Abstract
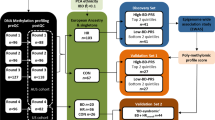
Methylation-specific (MS) multiplex ligation-dependent probe amplification (MLPA) at two differentially methylated regions (DMRs) at chromosome 11p15, H19-DMR and LIT1-DMR, and microsatellite analysis for uniparental disomy (UPD) at chromosome 7 or 11, have been recommended for the genetic diagnosis of the Beckwith–Wiedemann syndrome (BWS) and the Silver–Russell syndrome (SRS). In this study, the efficacy of the MS pyrosequencing method at H19-DMR and LIT1-DMR at 11p15 and SGCE-DMR at 7q21 was evaluated for the genetic diagnosis of BWS (n=18) and SRS (n=20) patients. Epigenetic alterations or UPD were detected in 83% of BWS and 50% of SRS individuals by MS-MLPA, but the detection rate increased to 95% of BWS and 70% of SRS by MS pyrosequencing. Thirteen BWS patients (72%) harbored loss-of-methylation (LOM) at LIT1-DMR and two patients (11%) harbored gain-of-methylation (GOM) at H19-DMR, whereas two patients (11%) had both LOM at LIT1-DMR and GOM at H19-DMR, reflecting paternal UPD 11. Thirteen SRS patients (65%) harbored LOM at H19-DMR, whereas one patient (5%) had GOM at SGCE-DMR, reflecting maternal UPD 7. Birth anthropometric profiles were significantly correlated to methylation scores at either H19-DMR or LIT1-DMR. In conclusion, MS pyrosequencing enhanced the detection rate of molecular defects in BWS and SRS. Moreover, it indicates that methylation status at 11p15.5 might have an important role in fetal growth.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Choufani, S., Shuman, C. & Weksberg, R. Beckwith-Wiedemann syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 154C, 343–354 (2010).
Eggermann, T. Russell-Silver syndrome. Am. J. Med. Genet. C. Semin. Med. Genet. 154C, 355–364 (2010).
Hao, Y., Crenshaw, T., Moulton, T., Newcomb, E. & Tycko, B. Tumour-suppressor activity of H19 RNA. Nature 365, 764–767 (1993).
Lee, M. P., DeBaun, M. R., Mitsuya, K., Galonek, H. L., Brandenburg, S., Oshimura, M. et al. Loss of imprinting of a paternally expressed transcript, with antisense orientation to KVLQT1, occurs frequently in Beckwith-Wiedemann syndrome and is independent of insulin-like growth factor II imprinting. Proc. Natl Acad. Sci. USA 96, 5203–5208 (1999).
Matsuoka, S., Edwards, M. C., Bai, C., Parker, S., Zhang, P., Baldini, A. et al. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 9, 650–662 (1995).
Gicquel, C., Rossignol, S., Cabrol, S., Houang, M., Steunou, V., Barbu, V. et al. Epimutation of the telomeric imprinting center region on chromosome 11p15 in Silver-Russell syndrome. Nat. Genet. 37, 1003–1007 (2005).
Gaston, V., Le Bouc, Y., Soupre, V., Burglen, L., Donadieu, J., Oro, H. et al. Analysis of the methylation status of the KCNQ1OT and H19 genes in leukocyte DNA for the diagnosis and prognosis of Beckwith-Wiedemann syndrome. Eur. J. Hum. Genet. 9, 409–418 (2001).
Engel, J. R., Smallwood, A., Harper, A., Higgins, M. J., Oshimura, M., Reik, W. et al. Epigenotype-phenotype correlations in Beckwith-Wiedemann syndrome. J. Med. Genet. 37, 921–926 (2000).
Li, M., Squire, J., Shuman, C., Fei, Y. L., Atkin, J., Pauli, R. et al. Imprinting status of 11p15 genes in Beckwith-Wiedemann syndrome patients with CDKN1C mutations. Genomics 74, 370–376 (2001).
Lee, M. P., DeBaun, M., Randhawa, G., Reichard, B. A., Elledge, S. J. & Feinberg, A. P. Low frequency of p57KIP2 mutation in Beckwith-Wiedemann syndrome. Am. J. Hum. Genet. 61, 304–309 (1997).
Abu-Amero, S., Monk, D., Frost, J., Preece, M., Stanier, P. & Moore, G. E. The genetic aetiology of Silver-Russell syndrome. J. Med. Genet. 45, 193–199 (2008).
Petry, C. J., Seear, R. V., Wingate, D. L., Acerini, C. L., Ong, K. K., Hughes, I. A. et al. Maternally transmitted foetal H19 variants and associations with birth weight. Hum. Genet. 130, 663–670 (2011).
Penaherrera, M. S., Weindler, S., Van Allen, M. I., Yong, S. L., Metzger, D. L., McGillivray, B. et al. Methylation profiling in individuals with Russell-Silver syndrome. Am. J. Med. Genet. A 152A, 347–355 (2010).
Azzi, S., Rossignol, S., Steunou, V., Sas, T., Thibaud, N., Danton, F. et al. Multilocus methylation analysis in a large cohort of 11p15-related foetal growth disorders (Russell Silver and Beckwith Wiedemann syndromes) reveals simultaneous loss of methylation at paternal and maternal imprinted loci. Hum. Mol. Genet. 18, 4724–4733 (2009).
Wiedemann, H. R. Familial malformation complex with umbilical hernia and macroglossia–a ‘new syndrome’? J. Genet. Hum. 13, 223–232 (1964).
Shuman, C., Beckwith, J. B., Smith, A. C. & Weksberg, R. in GeneReviews(Pagon R. A., Bird T. D., Dolan C. R., Stephens K., Adam M. P. ) (University of Washington, Seattle: Seattle, WA, 1993).
Saal, H. M., Pagon, R. A. & Pepin, M. G. Reevaluation of Russell-Silver syndrome. J. Pediatr. 107, 733–737 (1985).
Herman, J. G., Graff, J. R., Myohanen, S., Nelkin, B. D. & Baylin, S. B. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl Acad. Sci. USA 93, 9821–9826 (1996).
Niklasson, A. & Albertsson-Wikland, K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 8, 8 (2008).
Guo, L., Choufani, S., Ferreira, J., Smith, A., Chitayat, D., Shuman, C. et al. Altered gene expression and methylation of the human chromosome 11 imprinted region in small for gestational age (SGA) placentae. Dev. Biol. 320, 79–91 (2008).
Wakeling, E. L., Amero, S. A., Alders, M., Bliek, J., Forsythe, E., Kumar, S. et al. Epigenotype-phenotype correlations in Silver-Russell syndrome. J. Med. Genet. 47, 760–768 (2010).
Bliek, J., Maas, S. M., Ruijter, J. M., Hennekam, R. C., Alders, M., Westerveld, A. et al. Increased tumour risk for BWS patients correlates with aberrant H19 and not KCNQ1OT1 methylation: occurrence of KCNQ1OT1 hypomethylation in familial cases of BWS. Hum. Mol. Genet. 10, 467–476 (2001).
Bourque, D. K., Penaherrera, M. S., Yuen, R. K., Van Allen, M. I., McFadden, D. E. & Robinson, W. P. The utility of quantitative methylation assays at imprinted genes for the diagnosis of fetal and placental disorders. Clin. Genet. 79, 169–175 (2010).
Bliek, J., Verde, G., Callaway, J., Maas, S. M., De Crescenzo, A., Sparago, A. et al. Hypomethylation at multiple maternally methylated imprinted regions including PLAGL1 and GNAS loci in Beckwith-Wiedemann syndrome. Eur. J. Hum. Genet. 17, 611–619 (2009).
Azzi, S., Rossignol, S., Le Bouc, Y. & Netchine, I. Lessons from imprinted multilocus loss of methylation in human syndromes: a step toward understanding the mechanisms underlying these complex diseases. Epigenetics 5, 373–377 (2010).
Meyer, E., Lim, D., Pasha, S., Tee, L. J., Rahman, F., Yates, J. R. et al. Germline mutation in NLRP2 (NALP2) in a familial imprinting disorder (Beckwith-Wiedemann Syndrome). PLoS Genet. 5, e1000423 (2009).
Monk, D., Bentley, L., Hitchins, M., Myler, R. A., Clayton-Smith, J., Ismail, S. et al. Chromosome 7p disruptions in Silver Russell syndrome: delineating an imprinted candidate gene region. Hum. Genet. 111, 376–387 (2002).
Kim, Y., Kim, S. S., Kim, G., Park, S., Park, I. S. & Yoo, H. W. Detection of maternal uniparental disomy at the two imprinted genes on chromosome 7, GRB10 and PEG1/MEST, in a Silver-Russell syndrome patient using methylation-specific PCR assays. Clin. Genet. 67, 267–269 (2005).
Acknowledgements
We appreciate the patients and families for their cooperation. This research was supported by a grant from the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (grant number NRF-2011-0019674).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Lee, B., Kim, GH., Oh, T. et al. Quantitative analysis of methylation status at 11p15 and 7q21 for the genetic diagnosis of Beckwith–Wiedemann syndrome and Silver–Russell syndrome. J Hum Genet 58, 604–610 (2013). https://doi.org/10.1038/jhg.2013.67
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2013.67
Keywords
This article is cited by
-
Clinical and molecular features of children with Beckwith-Wiedemann syndrome in China: a single-center retrospective cohort study
Italian Journal of Pediatrics (2020)
-
Primordial dwarfism: overview of clinical and genetic aspects
Molecular Genetics and Genomics (2016)
-
A multi-method approach to the molecular diagnosis of overt and borderline 11p15.5 defects underlying Silver–Russell and Beckwith–Wiedemann syndromes
Clinical Epigenetics (2016)
-
European guidance for the molecular diagnosis of pseudohypoparathyroidism not caused by point genetic variants at GNAS: an EQA study
European Journal of Human Genetics (2015)