Abstract
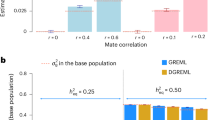
Comparing genetic and phenotypic similarity among unrelated individuals seems a promising way to quantify the genetic component of traits while avoiding the problematic assumptions plaguing twin- and other kin-based estimates of heritability. One approach uses a Genetic Relatedness Estimation through Maximum Likelihood (GREML) model for individuals who are related at less than 0.025 to predict their phenotypic similarity by their genetic similarity. Here we test the key underlying assumption of this approach: that genetic relatedness is orthogonal to environmental similarity. Using data from the Health and Retirement Study (and two other surveys), we show two unrelated individuals may be more likely to have been reared in a similar environment (urban versus nonurban setting) if they are genetically similar. This effect is not eliminated by controls for population structure. However, when we include this environmental confound in GREML models, heritabilities do not change substantially and thus potential bias in estimates of most biological phenotypes is probably minimal.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
25 June 2014
This article has been corrected since Advance Online Publication, and a corrigendum is also printed in this issue.
References
Breen, F., Plomin, R. & Wardle, J. Heritability of food preferences in young children. Physiol. Behav. 88, 443–447 (2006).
Rodgers, J. L., Kohler, H. P., Kyvik, K. O. & Christensen, K. Behavior genetic modeling of human fertility: findings from a contemporary Danish twin study. Demography 38, 29–42 (2001).
Van den Oord, E. J. A study of genetic and environmental effects on the co-occurrence of problem behaviors in three-year-old-twins. J. Abnorm. Psychol. 109, 360 (2000).
Rodgers, J., Rowe, D. & Buster, M. Nature, nurture and first sexual intercourse in the USA: fitting behavioural genetic models to NLSY kinship data. J. Biosoc. Sci. 31, 29–41 (1999).
Allison, D. B., Kaprio, J., Korkeila, M., Koskenvuo, M., Neale, M. C. & Hayakawa, K. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int. J. Obes. 20, 501–506 (1996).
Plomin, R., Owen, M. & McGuffin, P. The genetic basis of complex human behaviors. Science 264, 1733–1739 (1994).
Purcell, S. Variance components models for geneenvironment interaction in quantitative trait locus linkage analysis. Twin Res. 5, 572–576 (2002).
Goldberger, A. Heritability. Economica 46, 327–347 (1979).
Scarr, S. & Carter-Saltzman, L. Twin method: defense of a critical assumption. Behav. Genet. 9, 527–542 (1979).
Purcell, S., Wray, N. R., Stone, J. L., Visscher, P. M., O’Donovan, M. C., Sullivan, P. F. et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752 (2009).
Yang, J., Benyamin, B. & McEvoy, B. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 42, 565–569 (2010).
Yang, J., Lee, S., Goddard, M. & Visscher, P. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Davies, G., Tenesa, A., Payton, A., Yang, J., Harris, S. E., Liewald, D. et al. Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Mol. Psychiatry 16, 996–1005 (2011).
Belsky, D. W., Sears, M. R., Hancox, R. J., Harrington, H., Houts, R., Moffitt, T. E. et al. Polygenic risk and the development and course of asthma: an analysis of data from a four-decade longitudinal study. Lancet Respir. Med. 1, 453–461 (2013).
Belsky, D. W., Moffitt, T. E., Baker, T. B., Biddle, A. K., Evans, J. P., Harrington, H. et al. Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: evidence from a 4-decade longitudinal study. JAMA Psychiatry 70, 534–542 (2013).
Belsky, D. W., Moffitt, T. E., Houts, R., Bennett, G. G., Biddle, A. K., Blumenthal, J. A. et al. Polygenic risk, rapid childhood growth, and the development of obesity: evidence from a 4-decade longitudinal study. Arch. Pediatr. Adolesc. Med. 166, 515–521 (2012).
Rietveld, C., Medland, S., Derringer, J. & Yang, J. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science 340, 1467–1471 (2013).
Benjamin, D. J., Cesarini, D., van der Loos, M. J., Dawes, C. T., Koellinger, P. D., Magnusson, P. K. et al. The genetic architecture of economic and political preferences. Proc. Natl Acad. Sci. USA 109, 8026–8031 (2012).
Krabbendam, L. & Van Os, J. Schizophrenia and urbanicity: a major environmental influence—conditional on genetic risk. Schizophr. Bull. 31, 795–799 (2005).
Stefanis, N., Delespaul, P., Smyrnis, N., Lembesi, A., Avramopoulos, D. A., Evdokimidis, I. K. et al. Is the excess risk of psychosis-like experiences in urban areas attributable to altered cognitive development? Soc. Psychiatry 39, 364–368 (2004).
Spauwen, J., Krabbendam, L., Lieb, R., Wittchen, H. U. & Van Os, J. Evidence that the outcome of developmental expression of psychosis is worse for adolescents growing up in an urban environment. Psychol. Med. 36, 407–415 (2006).
Priftis, K., Anthracopoulos, M. B., Nikolaou-Papanagiotou, A., Matziou, V., Paliatsos, A. G., Tzavelas, G. et al. Increased sensitization in urban vs. rural environment–rural protection or an urban living effect? Pediatr. Allergy Immunol. 18, 209–216 (2007).
Jencks, C. & Mayer, S. in Inn. poverty United States (1990). http://www.books.google.com/books?hl=en&lr=&id=P7IV4eaGcxwC&oi=fnd&pg=PA111&dq=The+social+consequences+of+growing+up+in+poor+city+neighborhood&ots=Zn2Q7Z3SD4&sig=CxdXMCb0dKjkt1zrCr-6Av_UnwA.
Price, A. L., Patterson, N. J., Plenge, R. M., Weinblatt, M. E., Shadick, N. A. & Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Price, A., Zaitlen, N., Reich, D. & Patterson, N. New approaches to population stratification in genome-wide association studies. Nat. Rev. Genet. 11, 459–463 (2010).
Kang, H. M., Sul, J. H., Service, S. K., Zaitlen, N. A., Kong, S. Y., Freimer, N. B. et al. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 42, 348–354 (2010).
Manichaikul, A., Mychaleckyj, J. C., Rich, S. S., Daly, K., Sale, M. & Chen, W. M. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873 (2010).
Acknowledgements
This research uses data from The National Longitudinal Study of Adolescent Health (Add Health), a program project directed by Kathleen Mullan Harris and designed by J Richard Udry, Peter S Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Information on how to obtain the Add Health data files is available on the Add Health website (http://www.cpc.unc.edu/addhealth). The Framingham Heart Study (FHS; accession #7909-7) was supported by the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine, and the National Heart, Lung and Blood Institute's Framingham Heart Study. The Health and Retirement Study (HRS; accession number 0925-0670) is sponsored by the National Institute on Aging (grant numbers NIA U01AG009740, RC2AG036495, and RC4AG039029) and is conducted by the University of Michigan. Additional funding support for genotyping and analysis were provided by NIH/NICHD R01 HD060726.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Conley, D., Siegal, M., W Domingue, B. et al. Testing the key assumption of heritability estimates based on genome-wide genetic relatedness. J Hum Genet 59, 342–345 (2014). https://doi.org/10.1038/jhg.2014.14
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2014.14
Keywords
This article is cited by
-
Genetic variation, brain, and intelligence differences
Molecular Psychiatry (2022)
-
Genomic analysis of family data reveals additional genetic effects on intelligence and personality
Molecular Psychiatry (2018)
-
Cohort Effects in the Genetic Influence on Smoking
Behavior Genetics (2016)