Abstract
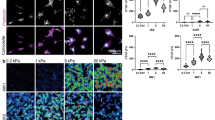
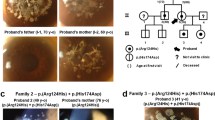
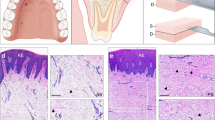
Although extraocular muscles are commonly affected by myasthenia gravis (MG) at presentation, a treatment-resistant ophthalmoplegic complication of MG (OP-MG) occurs in younger patients with African-genetic ancestry. In MG, pathogenic antibodies activate complement-mediated muscle damage and this may be potentiated in some OP-MG cases because of relative deficiency of decay-accelerating factor/CD55. Extending this argument, we hypothesized that OP-MG individuals may harbor African-specific polymorphisms in key genes influencing extraocular muscle remodeling. We screened the regulatory region of the transforming growth factor beta-1 (TGFB1) gene encoding the cytokine pivotal in muscle healing responses. We show the frequency of an African-specific polymorphism TGFB1 c.−387 T (rs11466316) among South Africans with African-genetic ancestry is higher than 1000 Genomes African controls (17.2% vs 4.8%; P<1 × 10−7), and associates with juvenile OP-MG (28%; P=0.043). Further, TGFB1 −387 C>T is functional because it represses the TGFB1 promoter construct basal activity by fivefold, and OP-MG fibroblasts (−387 C/T or T/T) have lower basal TGFB1 mRNA transcripts compared with controls (−387 C/C)(P=0.001). Co-transfections with Sp1 show less responsiveness of the −387 T promoter compared with wild-type −387 C (P=0.015). Our findings suggest that population-specific alleles may lower TGFB1 expression, thereby influencing OP-MG susceptibility by inhibiting extraocular muscle CD55 upregulation and/or altered endplate remodeling.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Soltys, J., Gong, B., Kaminski, H. J., Zhou, Y. & Kusner, L. L. Extraocular muscle susceptibility to myasthenia gravis: unique immunological environment? Ann. NY Acad. Sci. 1132, 220–224 (2008).
Heckmann, J. M., Owen, E. P. & Little, F. Myasthenia gravis in South Africans: racial differences in clinical manifestations. Neuromuscul. Disord. 17, 929–934 (2007).
Heckmann, J. M., Hansen, P., Van Toorn, R., Lubbe, E., Janse van Rensburg, E. & Wilmshurst, J. M. The characteristics of juvenile myasthenia gravis among South Africans. S. Afr. Med. J. 102, 532–536 (2012).
Biesecker, G. & Gomez, C. M. Inhibition of acute passive transfer experimental autoimmune myasthenia gravis with Fab antibody to complement C6. J. Immunol. 142, 2654–2659 (1989).
Lennon, V. A., Seybold, M. E., Lindstrom, J. M., Cochrane, C. & Ulevitch, R. Role of complement in the pathogenesis of experimental autoimmune myasthenia gravis. J. Exp. Med. 147, 973–983 (1978).
Morgan, B. P., Chamberlain-Banoub, J., Neal, J. W., Song, W., Mizuno, M. & Harris, C. L. The membrane attack pathway of complement drives pathology in passively induced experimental autoimmune myasthenia gravis in mice. Clin. Exp. Immunol. 146, 294–302 (2006).
Sahashi, K., Engel, A. G., Lambert, E. H. & Howard, F. M. Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J. Neuropathol. Exp. Neurol. 39, 160–172 (1980).
Kaminski, H. J., Kusner, L. L., Richmonds, C., Medof, M. E. & Lin, F. Deficiency of decay accelerating factor and CD59 leads to crisis in experimental myasthenia. Exp. Neurol. 202, 287–293 (2006).
Kaminski, H. J., Li, Z., Richmonds, C., Lin, F. & Medof, M. E. Complement regulators in extraocular muscle and experimental autoimmune myasthenia gravis. Exp. Neurol. 189, 333–342 (2004).
Lin, F., Kaminski, H. J., Conti-Fine, B. M., Wang, W., Richmonds, C. & Medof, M. E. Markedly enhanced susceptibility to experimental autoimmune myasthenia gravis in the absence of decay-accelerating factor protection. J. Clin. Invest. 110, 1269–1274 (2002).
Medof, M. E., Kinoshita, T. & Nussenzweig, V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J. Exp. Med. 160, 1558–1578 (1984).
Soltys, J., Halperin, J. & Xuebin, Q. DAF/CD55 and Protectin/CD59 modulate adaptive immunity and disease outcome in experimental autoimmune myasthenia gravis. J. Neuroimmunol. 244, 63–69 (2012).
Porter, J. D., Khanna, S., Kaminski, H. J., Rao, J. S., Merriam, P., Richmonds, C. R. et al. Extraocular muscle is defined by a fundamentally distinct gene expression profile. Proc. Natl Acad. Sci. USA 98, 12062–12067 (2001).
Zhou, Y., Kaminski, H. J., Gong, B., Cheng, G., Feuerman, J. M. & Kusner, L. RNA expression analysis of passive transfer myasthenia supports extraocular muscle as a unique immunological environment. Invest. Ophthalmol. Vis. Sci. 55, 4348–4359 (2014).
Heckmann, J. M., Uwimpuhwe, H., Ballo, R., Kaur, M., Bajic, V. B. & Prince, S. A functional SNP in the regulatory region of the decay-accelerating factor gene associates with extraocular muscle pareses in myasthenia gravis. Genes Immun. 11, 1–10 (2010).
Auret, J., Abrahams, A., Prince, S. & Heckmann, J. M. The effects of prednisone and steroid-sparing agents on decay accelerating factor (CD55) expression: implications in myasthenia gravis. Neuromuscul. Disord. 24, 499–508 (2014).
Burks, T. N. & Cohn, R. D. Role of TGF-β signaling in inherited and acquired myopathies. Skelet. Muscle 1, 1–13 (2011).
Li, Y., Foster, W., Deasy, B. M., Chan, Y., Prisk, V., Tang, Y. et al. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am. J. Pathol. 164, 1007–1019 (2004).
Mendias, C. L., Gumucio, J. P., Davis, M. E., Bromley, C. W., Davis, C. S. & Brooks, S. V. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve 45, 55–59 (2012).
Porter, J. D., Israel, S., Gong, B., Merriam, A. P., Feuerman, J., Khanna, S. et al. Distinctive morphological and gene/protein expression signatures during myogenesis in novel cell lines from extraocular and hindlimb muscle. Physiol. Genomics 24, 264–275 (2006).
Shah, R., Rahaman, B., Hurley, C. K. & Posch, P. E. Allelic diversity in the TGFB1 regulatory region: characterization of novel functional single nucleotide polymorphisms. Hum. Genet. 119, 61–74 (2006).
Mombaur, B., Lesosky, M. R., Liebenberg, L., Vreede, H. & Heckmann, J. M. Incidence of acetylcholine receptor-antibody-positive myasthenia gravis in South Africa. Muscle Nerve 51, 533–537 (2015).
May, A., Hazelhurst, S., Li, Y., Norris, S., Govind, N., Tikly, M. et al. Genetic diversity in black South Africans from Soweto. BMC Genomics 14, 1–12 (2013).
Barrett, J. C, Fry, B., Maller, J. & Daly, M. J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005).
Gabriel, S. B., Schaffner, S. F., Nguyen, H., Moore, J. M., Roy, J., Blumenstiel, B. et al. The structure of haplotype blocks in the human genome. Science 296, 2225–2229 (2002).
Nyholt, D. R. A simple correction for multiple testing for single-nucleotide polymorphisms in linage disequilibrium with each other. Am. J. Hum. Genet. 74, 765–769 (2004).
Healy, J., Dionne, J., Bélanger, H., Larivière, M., Beaulieu, P., Labuda, D. et al. Functional impact of sequence variation in the promoter region of TGFB1. Int. J. Cancer 125, 1483–1489 (2009).
Shah, R., Hurley, C. K. & Posch, P. E. A molecular mechanism for the differential regulation of TGF-beta1 expression due to the common SNP −509C-T (c. −1347C>T). Hum. Genet 120, 461–469 (2006).
Walker, G. E., Wilson, E. M., Powell, D. & Oh, Y. Butyrate, a histone deacetylase inhibitor, activates the human IGF binding protein-3 promoter in breast cancer cells: Molecular mechanism involves an Sp1/Sp3 multiprotein complex. Endocrinology 142, 3817–3827 (2001).
Yang, J., Kawai, Y., Hanson, R. W. & Arinze, I. J. Sodium butyrate induces transcription from the Gαi2 gene promoter through multiple Sp1 sites in the promoter and by activating the MEK-ERK signal transduction pathway. J. Biol. Chem. 276, 25742–25752 (2001).
Barnard, J. A. & Warwick, G. Butyrate rapidly induces growth inhibition and differentiation in HT-29 cells. Cell Growth Differ. 4, 495–501 (1993).
Skeen, V. R., Collard, T. J., Southern, S. L., Greenhough, A., Hague, A., Townsend, P. A et al. BAG-1 suppresses expression of the key regulatory cytokine transforming growth factor β (TGF-β1) in colorectal tumour cells. Oncogene 32, 4490–4499 (2012).
Knight, J. Approaches for establishing the function of regulatory genetic variants involved in disease. Genome Med 6, 1–15 (2014).
Schuster, S. C., Miller, W., Ratan, A., Tomsho, L. P., Giardine, B., Kasson, L. R. et al. Complete Khoisan and Bantu genomes from southern Africa. Nature 463, 943–947 (2010).
Tishkoff, S. A., Reed, F. A., Friedlaender, F. R., Ehret, C., Ranciaro, A., Froment, A. et al. The genetic structure and history of Africans and African Americans. Science 324, 1035–1044 (2009).
Davie, J. R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 133, 2485 S–2493 S (2003).
Matsumoto, N., Riley, S., Fraser, D., Al-Assaf, S., Ishimura, E., Wolever, T. et al. Butyrate modulates TGF-β1 generation and function: potential renal benefit for Acacia(sen) SUPERGUMTM (gum arabic)? Kidney Int. 69, 257–265 (2006).
Cocuzzi, E. T., Bardenstein, D. S., Stavitsky, A., Sundarraj, N. & Medof, M. E. Upregulation of DAF (CD55) on orbital fibroblasts by cytokines. Differential effects of TNF-beta and TNF-alpha. Curr. Eye Res. 23, 86–92 (2001).
Acknowledgements
MN received a University of Cape Town Neurology fellowship and a South African Medical Research Grant (MRC) Post-Intern scholarship, JMH received support from the South African MRC, RR received a Discovery fellowship, J-MB a National Research Foundation (NRF) Scholarship, and SP received support from the MRC and NRF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Nel, M., Buys, JM., Rautenbach, R. et al. The African−387 C>T TGFB1 variant is functional and associates with the ophthalmoplegic complication in juvenile myasthenia gravis. J Hum Genet 61, 307–316 (2016). https://doi.org/10.1038/jhg.2015.146
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2015.146
This article is cited by
-
Gene expression profiling of orbital muscles in treatment-resistant ophthalmoplegic myasthenia gravis
Orphanet Journal of Rare Diseases (2020)
-
The functionality of African-specific variants in the TGFB1 regulatory region and their potential role in HIVAN
Clinical and Experimental Nephrology (2018)
-
RETRACTED ARTICLE: Juvenile-onset myasthenia gravis: autoantibody status, clinical characteristics and genetic polymorphisms
Journal of Neurology (2017)