Abstract
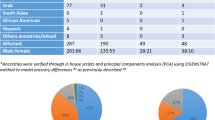
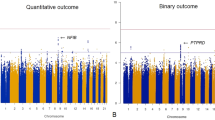
Many patients with schizophrenia have poor clinical and social outcomes. Some risk alleles closely related to the onset of schizophrenia have been reported to be associated with their clinical phenotypes, but the direct relationship between genetic vulnerability to schizophrenia and clinical/social outcomes of schizophrenia, as evaluated by both practical clinical scales and ‘real-world’ function, has not been investigated. We evaluated the clinical and social outcomes of 455 Japanese patients with schizophrenia by severity of illness according to the Clinical Global Impression-Severity Scale (CGI-S) and social outcomes by social adjustment/maladjustment at 5 years after the first visit. We examined whether 46 single nucleotide polymorphisms (SNPs) selected from a Japanese genome-wide association study of susceptibility to schizophrenia were associated with clinical and social outcomes. We also investigated the polygenic risk scores of 46 SNPs. Allele-wise association analysis detected three SNPs, including rs2623659 in the CUB and Sushi multiple domains-1 (CSMD1) gene, associated with severity of illness at end point. The severity of illness at end point was associated with treatment response, but not with the severity of illness at baseline. Three SNPs, including rs2294424 in the C6orf105 gene, were associated with social outcomes. Point estimates of odds ratios showed positive relationships between polygenic risk scores and clinical/social outcomes; however, the results were not statistically significant. Because these results are exploratory, we need to replicate them with a larger sample in a future study.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mueser, K. T. & McGurk, S. R. Schizophrenia. Lancet. 363, 2063–2072 (2004).
Meltzer, H. Y. Clozapine: balancing safety with superior antipsychotic efficacy. Clin. Schizophr. Relat. Psychoses 6, 134–144 (2012).
Robinson, D. G., Woerner, M. G., McMeniman, M., Mendelowitz, A. & Bilder, R. M. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am. J. Psychiatry. 161, 473–479 (2004).
Wiersma, D., Wanderling, J., Dragomirecka, E., Ganev, K., Harrison, G., An Der Heiden, W. et al. Social disability in schizophrenia: its development and prediction over 15 years in incidence cohorts in six European centres. Psychol. Med. 30, 1155–1167 (2000).
Mohr, P., Rodriguez, M., Bravermanova, A., Melicher, T., Ceplova, Z., Cermak, J. et al. Social and functional capacity of schizophrenia patients: a cross-sectional study. Int. J. Soc. Psychiatry. 60, 352–358 (2013).
Sado, M., Inagaki, A., Koreki, A., Knapp, M., Kissane, L. A., Mimura, M. et al. The cost of schizophrenia in Japan. Neuropsychiatr. Dis. Treat. 9, 787–798 (2013).
Cardno, A. G. & Gottesman, II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am. J. Med. Genet. 97, 12–17 (2000).
Cardno, A. G., Marshall, E. J., Coid, B., Macdonald, A. M., Ribchester, T. R., Davies, N. J. et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch. Gen. Psychiatry. 56, 162–168 (1999).
Singh, S., Kumar, A., Agarwal, S., Phadke, S. R. & Jaiswal, Y. Genetic insight of schizophrenia: past and future perspectives. Gene 535, 97–100 (2014).
O'Donovan, M. C., Craddock, N. J. & Owen, M. J. Genetics of psychosis; insights from views across the genome. Hum. Genet. 126, 3–12 (2009).
Cross-Disorder Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
Hashimoto, R., Ohi, K., Yasuda, Y., Fukumoto, M., Iwase, M., Iike, N. et al. The impact of a genome-wide supported psychosis variant in the ZNF804A gene on memory function in schizophrenia. Am. J. Med. Genet. B 153B, 1459–1464 (2010).
Wassink, T. H., Epping, E. A., Rudd, D., Axelsen, M., Ziebell, S., Fleming, F. W. et al. Influence of ZNF804a on brain structure volumes and symptom severity in individuals with schizophrenia. Arch. Gen. Psychiatry. 69, 885–892 (2012).
Ohi, K., Hashimoto, R., Yasuda, Y., Fukumoto, M., Yamamori, H., Umeda-Yano, S. et al. Influence of the NRGN gene on intellectual ability in schizophrenia. J. Hum. Genet. 58, 700–705 (2013).
Stefansson, H., Ophoff, R. A., Steinberg, S., Andreassen, O. A., Cichon, S., Rujescu, D. et al. Common variants conferring risk of schizophrenia. Nature 460, 744–747 (2009).
Thong, J. Y., Qiu, A., Sum, M. Y., Kuswanto, C. N., Tuan, T. A., Donohoe, G. et al. Effects of the neurogranin variant rs12807809 on thalamocortical morphology in schizophrenia. PLoS ONE 8, e85603 (2013).
Miyatake, S., Touho, H., Miyake, N., Ohba, C., Doi, H., Saitsu, H. et al. Sibling cases of moyamoya disease having homozygous and heterozygous c.14576G>A variant in RNF213 showed varying clinical course and severity. J. Hum. Genet. 57, 804–806 (2012).
Onouchi, Y., Gunji, T., Burns, J. C., Shimizu, C., Newburger, J. W., Yashiro, M. et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat. Genet. 40, 35–42 (2008).
Birchwood, M., Todd, P. & Jackson, C. Early intervention in psychosis. The critical period hypothesis. Br. J. Psychiatry Suppl. 172, 53–59 (1998).
Kane, J. M., Honigfeld, G., Singer, J. & Meltzer, H. Clozapine in treatment-resistant schizophrenics. Psychopharmacol. Bull. 24, 62–67 (1988).
Norman, R. M., Manchanda, R. & Windell, D. The prognostic significance of early remission of positive symptoms in first treated psychosis. Psychiatry Res. 218, 44–47 (2014).
Ikeda, M., Aleksic, B., Kinoshita, Y., Okochi, T., Kawashima, K., Kushima, I. et al. Genome-wide association study of schizophrenia in a Japanese population. Biol. Psychiatry. 69, 472–478 (2011).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Marwaha, S. & Johnson, S. Schizophrenia and employment - a review. Soc. Psychiatry Psychiatr. Epidemiol. 39, 337–349 (2004).
Suzuki, T., Remington, G., Mulsant, B. H., Uchida, H., Rajji, T. K., Graff-Guerrero, A. et al. Defining treatment-resistant schizophrenia and response to antipsychotics: a review and recommendation. Psychiatry Res. 197, 1–6 (2012).
Kraus, D. M., Elliott, G. S., Chute, H., Horan, T., Pfenninger, K. H., Sanford, S. D. et al. CSMD1 is a novel multiple domain complement-regulatory protein highly expressed in the central nervous system and epithelial tissues. J. Immunol. 176, 4419–4430 (2006).
Steen, V. M., Nepal, C., Ersland, K. M., Holdhus, R., Naevdal, M., Ratvik, S. M. et al. Neuropsychological deficits in mice depleted of the schizophrenia susceptibility gene CSMD1. PLoS ONE 8, e79501 (2013).
Shimizu, A., Asakawa, S., Sasaki, T., Yamazaki, S., Yamagata, H., Kudoh, J. et al. A novel giant gene CSMD3 encoding a protein with CUB and sushi multiple domains: a candidate gene for benign adult familial myoclonic epilepsy on human chromosome 8q23.3-q24.1. Biochem. Biophys. Res. Commun. 309, 143–154 (2003).
Glancy, M., Barnicoat, A., Vijeratnam, R., de Souza, S., Gilmore, J., Huang, S. et al. Transmitted duplication of 8p23.1-8p23.2 associated with speech delay, autism and learning difficulties. Eur. J. Hum. Genet. 17, 37–43 (2009).
Havik, B., Le Hellard, S., Rietschel, M., Lybaek, H., Djurovic, S., Mattheisen, M. et al. The complement control-related genes CSMD1 and CSMD2 associate to schizophrenia. Biol. Psychiatry. 70, 35–42 (2011).
Schizophrenia Psychiatric Genome-Wide Association Study Consortium Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 43, 969–976 (2011).
Cross-Disorder Group of the Psychiatric Genomics Consortium Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 381, 1371–1379 (2013).
Donohoe, G., Walters, J., Hargreaves, A., Rose, E. J., Morris, D. W., Fahey, C. et al. Neuropsychological effects of the CSMD1 genome-wide associated schizophrenia risk variant rs10503253. Genes Brain Behav. 12, 203–209 (2013).
Koiliari, E., Roussos, P., Pasparakis, E., Lencz, T., Malhotra, A., Siever, L. J. et al. The CSMD1 genome-wide associated schizophrenia risk variant rs10503253 affects general cognitive ability and executive function in healthy males. Schizophr. Res. 154, 42–47 (2014).
Yamada, K., Iwayama, Y., Hattori, E., Iwamoto, K., Toyota, T., Ohnishi, T. et al. Genome-wide association study of schizophrenia in Japanese population. PLoS ONE 6, e20468 (2011).
Lupu, C., Zhu, H., Popescu, N. I., Wren, J. D. & Lupu, F. Novel protein ADTRP regulates TFPI expression and function in human endothelial cells in normal conditions and in response to androgen. Blood 118, 4463–4471 (2011).
Wang, F., Xu, C. Q., He, Q., Cai, J. P., Li, X. C., Wang, D. et al. Genome-wide association identifies a susceptibility locus for coronary artery disease in the Chinese Han population. Nat. Genet. 43, 345–349 (2011).
Park, J. W., Cai, J., McIntosh, I., Jabs, E. W., Fallin, M. D., Ingersoll, R. et al. High throughput SNP and expression analyses of candidate genes for non-syndromic oral clefts. J. Med. Genet. 43, 598–608 (2006).
Fanous, A. H., Zhou, B., Aggen, S. H., Bergen, S. E., Amdur, R. L., Duan, J. et al. Genome-wide association study of clinical dimensions of schizophrenia: polygenic effect on disorganized symptoms. Am. J. Psychiatry. 169, 1309–1317 (2012).
Frank, J., Lang, M., Witt, S. H., Strohmaier, J., Rujescu, D., Cichon, S. et al. Identification of increased genetic risk scores for schizophrenia in treatment-resistant patients. Mol. Psychiatry. 20, 150–151 (2015).
Penttila, M., Miettunen, J., Koponen, H., Kyllonen, M., Veijola, J., Isohanni, M. et al. Association between the duration of untreated psychosis and short- and long-term outcome in schizophrenia within the Northern Finland 1966 Birth Cohort. Schizophr. Res. 143, 3–10 (2013).
Marshall, M., Lewis, S., Lockwood, A., Drake, R., Jones, P. & Croudace, T. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch. Gen. Psychiatry. 62, 975–983 (2005).
Perkins, D. O., Gu, H., Boteva, K. & Lieberman, J. A. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am. J. Psychiatry. 162, 1785–1804 (2005).
Shibata, H., Yamamoto, K., Sun, Z., Oka, A., Inoko, H., Arinami, T. et al. Genome-wide association study of schizophrenia using microsatellite markers in the Japanese population. Psychiatr. Genet. 23, 117–123 (2013).
Acknowledgements
This research was supported, in part, by JSPS KAKENHI grant number 25461731 and the Zikei Institute of Psychiatry (Okayama, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Sakamoto, S., Takaki, M., Okahisa, Y. et al. Individual risk alleles of susceptibility to schizophrenia are associated with poor clinical and social outcomes. J Hum Genet 61, 329–334 (2016). https://doi.org/10.1038/jhg.2015.153
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2015.153
This article is cited by
-
Association between depression in chronic phase and future clinical outcome of patients with schizophrenia
Psychopharmacology (2022)
-
The complement system in schizophrenia: where are we now and what’s next?
Molecular Psychiatry (2020)
-
Switching strategies for antipsychotic monotherapy in schizophrenia: a multi-center cohort study of aripiprazole
Psychopharmacology (2020)