Abstract
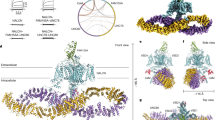
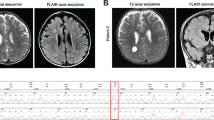
Three recessive mutations in the sodium leak channel, nonselective (NALCN) have been reported to cause intellectual disability and hypotonia. In addition, 14 de novo heterozygous mutations have been identified in 15 patients with arthrogryposis and neurodevelopmental impairment. Here, we report three patients with neurodevelopmental disease and hypotonia, harboring one recurrent (p.R1181Q) and two novel mutations (p.L312V and p.V1020F) occurring de novo in NALCN. Mutation p.L312 is located in the pore forming S6 region of domain I and p.V1020F in the S5 region of domain III. Mutation p.R1181Q is in a linker region. Mapping these three mutations to a model of NALCN showed p.Leu312 and p.Val1020 positioned in the hydrophobic core of the pore modules, indicating these two mutations may affect the gating function of NALCN. Although p.R1181Q is unlikely to affect the ion channel structure, previous studies have shown that an analogous mutation in Caenorhabditis elegans produced a phenotype with a coiling locomotion, suggesting that p.R1181Q could also affect NALCN function. Our three patients showed profound intellectual disability and growth delay, facial dysmorphologies and hypotonia. The present data support previous work suggesting heterozygous NALCN mutations lead to syndromic neurodevelopmental impairment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lu, B., Su, Y., Das, S., Liu, J., Xia, J. & Ren, D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell 129, 371–383 (2007).
Stephens, R. F., Guan, W., Zhorov, B. S. & Spafford, J. D. Selectivity filters and cysteine-rich extracellular loops in voltage-gated sodium, calcium, and NALCN channels. Front. Physiol. 6, 153 (2015).
Senatore, A. & Spafford, J. D. A uniquely adaptable pore is consistent with NALCN being an ion sensor. Channels 7, 60–68 (2013).
Ren, D. Sodium leak channels in neuronal excitability and rhythmic behaviors. Neuron 72, 899–911 (2011).
Koroglu, C., Seven, M. & Tolun, A. Recessive truncating NALCN mutation in infantile neuroaxonal dystrophy with facial dysmorphism. J. Med. Genet. 50, 515–520 (2013).
Al-Sayed, M. D., Al-Zaidan, H., Albakheet, A., Hakami, H., Kenana, R., Al-Yafee, Y. et al. Mutations in NALCN cause an autosomal-recessive syndrome with severe hypotonia, speech impairment, and cognitive delay. Am. J. Hum. Genet. 93, 721–726 (2013).
Mok, K. Y., Schneider, S. A., Trabzuni, D., Stamelou, M., Edwards, M., Kasperaviciute, D. et al. Genomewide association study in cervical dystonia demonstrates possible association with sodium leak channel. Mov. Disord. 29, 245–251 (2014).
Cochet-Bissuel, M., Lory, P. & Monteil, A. The sodium leak channel, NALCN, in health and disease. Front Cell Neurosci. 8, 132 (2014).
Chong, J. X., McMillin, M. J., Shively, K. M., Beck, A. E., Marvin, C. T., Armenteros, J. R. et al. De novo mutations in NALCN cause a syndrome characterized by congenital contractures of the limbs and face, hypotonia, and developmental delay. Am. J. Hum. Genet. 96, 462–473 (2015).
Aoyagi, K., Rossignol, E., Hamdan, F. F., Mulcahy, B., Xie, L., Nagamatsu, S. et al. A gain-of-function mutation in NALCN in a Child with intellectual disability, ataxia, and arthrogryposis. Hum. Mutat. 36, 753–757 (2015).
Fukai, R., Hiraki, Y., Yofune, H., Tsurusaki, Y., Nakashima, M., Saitsu, H. et al. A case of autism spectrum disorder arising from a de novo missense mutation in POGZ. J. Hum. Genet. 60, 277–279 (2015).
Ng, P. C. & Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 31, 3812–3814 (2003).
Adzhubei, I. A., Schmidt, S., Peshkin, L., Ramensky, V. E., Gerasimova, A., Bork, P. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Schwarz, J. M., Rodelsperger, C., Schuelke, M. & Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7, 575–576 (2010).
Strong, M., Chandy, K. G. & Gutman, G. A. Molecular evolution of voltage-sensitive ion channel genes: on the origins of electrical excitability. Mol. Biol. Evol. 10, 221–242 (1993).
Kelley, L. A. & Sternberg, M. J. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4, 363–371 (2009).
Kowal, J., Chami, M., Baumgartner, P., Arheit, M., Chiu, P. L., Rangl, M. et al. Ligand-induced structural changes in the cyclic nucleotide-modulated potassium channel MloK1. Nat. Commun. 5, 3106 (2014).
Payandeh, J., Scheuer, T., Zheng, N. & Catterall, W. A. The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358 (2011).
Zhang, X., Ren, W., DeCaen, P., Yan, C., Tao, X., Tang, L. et al. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature 486, 130–134 (2012).
Acknowledgements
We thank the patients and their families for participating in this work. We were supported in part by a grant for Research on Measures for Intractable Diseases, a grant for Comprehensive Research on Disability Health and Welfare, the Strategic Research Program for Brain Science (SRPBS) and a grant for Initiative on Rare and Undiagnosed Diseases in Pediatrics (IRUD-P) from the Japan Agency for Medical Research and Development (AMED); a Grant-in-Aid for Scientific Research on Innovative Areas (Transcription Cycle) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT); Grants-in-Aid for Scientific Research (A, B, and C), and challenging Exploratory Research from the Japan Society for the Promotion of Science (JSPS); the fund for Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems from the Japan Science and Technology Agency (JST); the Takeda Science Foundation; the Yokohama Foundation for Advancement of Medical Science; and the Hayashi Memorial Foundation for Female Natural Scientists.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Fukai, R., Saitsu, H., Okamoto, N. et al. De novo missense mutations in NALCN cause developmental and intellectual impairment with hypotonia. J Hum Genet 61, 451–455 (2016). https://doi.org/10.1038/jhg.2015.163
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2015.163
This article is cited by
-
Functional expression of CLIFAHDD and IHPRF pathogenic variants of the NALCN channel in neuronal cells reveals both gain- and loss-of-function properties
Scientific Reports (2019)
-
Methylation determines the extracellular calcium sensitivity of the leak channel NALCN in hippocampal dentate granule cells
Experimental & Molecular Medicine (2019)