Abstract
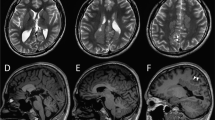
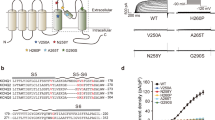
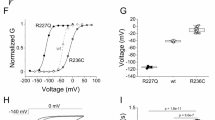
The voltage-gated Kv10.1 potassium channel, also known as ether-a-go-go-related gene 1, encoded by KCNH1 (potassium voltage-gated channel, subfamily H (eag related), member 1) is predominantly expressed in the central nervous system. Recently, de novo missense KCNH1 mutations have been identified in six patients with Zimmermann–Laband syndrome and in four patients with Temple–Baraitser syndrome. These syndromes were historically considered distinct. Here we report three de novo missense KCNH1 mutations in four patients with syndromic developmental delay and epilepsy. Two novel KCNH1 mutations (p.R357Q and p.R357P), found in three patients, were located at the evolutionally highly conserved arginine in the channel voltage-sensor domain (S4). Another mutation (p.G496E) was found in the channel pore domain (S6) helix, which acts as a hinge in activation gating and mainly conducts non-inactivating outward potassium current. A previously reported p.G496R mutation was shown to produce no voltage-dependent outward current in CHO cells, suggesting that p.G496E may also disrupt the proper function of the Kv channel pore. Our report confirms that KCNH1 mutations are associated with syndromic neurodevelopmental disorder, and also support the functional importance of the S4 domain.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gutman, G. A., Chandy, K. G., Grissmer, S., Lazdunski, M., McKinnon, D., Pardo, L. A. et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 57, 473–508 (2005).
Miceli, F., Soldovieri, M. V., Ambrosino, P., De Maria, M., Manocchio, L., Medoro, A. et al. Molecular pathophysiology and pharmacology of the voltage-sensing module of neuronal ion channels. Front Cell Neurosci. 9, 259 (2015).
Pardo, L. A., del Camino, D., Sánchez, A., Alves, F., Brüggemann, A., Beckh, S. et al. Oncogenic potential of EAG K(+) channels. EMBO J. 18, 5540–5547 (1999).
Haitin, Y., Carlson, A. E. & Zagotta, W. N. The structural mechanism of KCNH-channel regulation by the eag domain. Nature 501, 444–448 (2013).
Biervert, C., Schroeder, B. C., Kubisch, C., Berkovic, S. F., Propping, P., Jentsch, T. J. et al. A potassium channel mutation in neonatal human epilepsy. Science 279, 403–406 (1998).
Charlier, C., Singh, N. A., Ryan, S. G., Lewis, T. B., Reus, B. E., Leach, R. J. et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat. Genet. 18, 53–55 (1998).
Saitsu, H., Kato, M., Koide, A., Goto, T., Fujita, T., Nishiyama, K. et al. Whole exome sequencing identifies KCNQ2 mutations in Ohtahara syndrome. Ann. Neurol. 72, 298–300 (2012).
Weckhuysen, S., Mandelstam, S., Suls, A., Audenaert, D., Deconinck, T., Claes, L. R. et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 71, 15–25 (2012).
Muona, M., Berkovic, S. F., Dibbens, L. M., Oliver, K. L., Maljevic, S., Bayly, M. A. et al. A recurrent de novo mutation in KCNC1 causes progressive myoclonus epilepsy. Nat. Genet. 47, 39–46 (2015).
Simons, C., Rash, L. D., Crawford, J., Ma, L., Cristofori-Armstrong, B., Miller, D. et al. Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple-Baraitser syndrome and epilepsy. Nat. Genet. 47, 73–77 (2015).
Syrbe, S., Hedrich, U. B., Riesch, E., Djmié, T., Müller, S., Møller, R. S. et al. De novo loss- or gain-of-function mutations in KCNA2 cause epileptic encephalopathy. Nat. Genet. 47, 393–399 (2015).
Yang, Y., Vasylyev, D. V., Dib-Hajj, F., Veeramah, K. R., Hammer, M. F., Dib-Hajj, S. D. et al. Multistate structural modeling and voltage-clamp analysis of epilepsy/autism mutation Kv10.2-R327H demonstrate the role of this residue in stabilizing the channel closed state. J. Neurosci. 33, 16586–16593 (2013).
Ludwig, J., Terlau, H., Wunder, F., Brüggemann, A., Pardo, L. A., Marquardt, A. et al. Functional expression of a rat homologue of the voltage gated either á go-go potassium channel reveals differences in selectivity and activation kinetics between the Drosophila channel and its mammalian counterpart. EMBO J. 13, 4451–4458 (1994).
Gómez-Varela, D., Kohl, T., Schmidt, M., Rubio, M. E., Kawabe, H., Nehring, R. B. et al. Characterization of Eag1 channel lateral mobility in rat hippocampal cultures by single-particle-tracking with quantum dots. PLoS ONE 5, e8858 (2010).
Kortüm, F., Caputo, V., Bauer, C. K., Stella, L., Ciolfi, A., Alawi, M. et al. Mutations in KCNH1 and ATP6V1B2 cause Zimmermann-Laband syndrome. Nat. Genet. 47, 661–667 (2015).
Chen, Y., Sánchez, A., Rubio, M. E., Kohl, T., Pardo, L. A. & Stühmer, W. Functional K(v)10.1 channels localize to the inner nuclear membrane. PLoS ONE 6, e19257 (2011).
Stengel, R., Rivera-Milla, E., Sahoo, N., Ebert, C., Bollig, F., Heinemann, S. H. et al. Kcnh1 voltage-gated potassium channels are essential for early zebrafish development. J. Biol. Chem. 287, 35565–35575 (2012).
Börjesson, S. I. & Elinder, F. Structure, function, and modification of the voltage sensor in voltage-gated ion channels. Cell Biochem. Biophys. 52, 149–174 (2008).
Wynia-Smith, S. L., Gillian-Daniel, A. L., Satyshur, K. A. & Robertson, G. A. hERG gating microdomains defined by S6 mutagenesis and molecular modeling. J. Gen. Physiol. 132, 507–520 (2008).
Li, S. C. & Deber, C. M. Glycine and beta-branched residues support and modulate peptide helicity in membrane environments. FEBS Lett. 311, 217–220 (1992).
Beckstein, O., Biggin, P. C., Bond, P., Bright, J. N., Domene, C., Grottesi, A. et al. Ion channel gating: insights via molecular simulations. FEBS Lett. 555, 85–90 (2003).
Bright, J. N. & Sansom, M. S. P. The flexing/twirling helix: exploring the flexibility about molecular hinges formed by proline and glycine motifs in transmembrane helices. J. Phys. Chem. B 107, 627–636 (2003).
Ding, S., Ingleby, L., Ahern, C. A. & Horn, R. Investigating the putative glycine hinge in Shaker potassium channel. J. Gen. Physiol. 126, 213–226 (2005).
Acknowledgements
We thank the patients and their families for participating in this work. This work was supported in part by a grant for Research on Measures for Intractable Diseases, a grant for Comprehensive Research on Disability Health and Welfare, the Strategic Research Program for Brain Science, and a grant for Initiative on Rare and Undiagnosed Diseases in Pediatrics; a Grant-in-Aid for Scientific Research on Innovative Areas (Transcription Cycle) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; Grants-in-Aid for Scientific Research (A, B and C), and challenging Exploratory Research from the Japan Society for the Promotion of Science; the fund for Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems from the Japan Science and Technology Agency; the Takeda Science Foundation; the Yokohama Foundation for Advancement of Medical Science; and the Hayashi Memorial Foundation for Female Natural Scientists.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Fukai, R., Saitsu, H., Tsurusaki, Y. et al. De novo KCNH1 mutations in four patients with syndromic developmental delay, hypotonia and seizures. J Hum Genet 61, 381–387 (2016). https://doi.org/10.1038/jhg.2016.1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2016.1
This article is cited by
-
Establishment and characterization of ZJUCHi003: an induced pluripotent stem cell line from a patient with Temple–Baraitser/Zimmermann–Laband syndrome carrying KCNH1 c.1070G > A (p.R357Q) variant
Human Cell (2024)
-
Computational prognostic evaluation of Alzheimer’s drugs from FDA-approved database through structural conformational dynamics and drug repositioning approaches
Scientific Reports (2023)
-
Genetic architecture and phenotypic landscape of deafness and onychodystrophy syndromes
Human Genetics (2022)
-
Potassium Channel KCNH1 Activating Variants Cause Altered Functional and Morphological Ciliogenesis
Molecular Neurobiology (2022)
-
Syndromic disorders caused by gain-of-function variants in KCNH1, KCNK4, and KCNN3—a subgroup of K+ channelopathies
European Journal of Human Genetics (2021)