Abstract
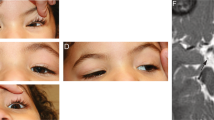
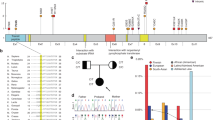
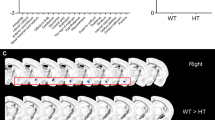
Spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disorder caused by survival motor neuron gene mutations. Variant forms of SMA accompanied by additional clinical presentations have been classified as atypical SMA and are thought to be caused by variants in as yet unidentified causative genes. Here, we presented the clinical findings of two siblings with an SMA variant followed by progressive cerebral atrophy, and the results of whole-exome sequencing analyses of the family quartet that was performed to identify potential causative variants. We identified two candidate homozygous missense variants, R942Q in the tubulin-folding cofactor D (TBCD) gene and H250Q in the bromo-adjacent homology domain and coiled-coil containing 1 (BAHCC1) gene, located on chromosome 17q25.3 with an interval of 1.4 Mbp. The in silico analysis of both variants suggested that TBCD rather than BAHCC1 was likely the pathogenic gene (TBCD sensitivity, 0.68; specificity, 0.97; BAHCC1 sensitivity, 1.00; specificity, 0.00). Thus, our results show that TBCD is a likely novel candidate gene for atypical SMA with progressive cerebral atrophy. TBCD is predicted to have important functions on tubulin integrity in motor neurons as well as in the central nervous system.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rudnik-Schöneborn, S., Goebel, H. H., Schlote, W., Molaian, S., Omran, H., Ketelsen, U. et al. Classical infantile spinal muscular atrophy with SMN deficiency causes sensory neuronopathy. Neurology 60, 983–987 (2003).
Pierre, L. & Jonathan, B. Early onset (childhood) monogenic neuropathies. Handb. Clin. Neurol. 115, 863–891 (2013).
Pierre, L., Jonathan, B. & Peter, D. Hereditary motor-sensory, motor, and sensory neuropathies in childhood. Handb. Clin. Neurol. 113, 1413–1432 (2013).
Wirth, B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum. Mutat. 15, 228–237 (2000).
Ursula, F. M., Katja, G., Anja, H., Christine, S., Klaus, Z., Christoph, B. et al. Severe spinal muscular atrophy variant associated with congenital bone fractures. J. Child. Neurol. 17, 718–721 (2002).
Klaus, Z. & Sabine, R. S. 93rd ENMC international workshop: non-5q-spinal muscular atrophies (SMA) – clinical picture (6–8 April 2001, Naarden, The Netherlands). Neuromuscul. Disord. 13, 179–183 (2003).
Nathalie, G., Jean-Marie, C., Jean-Christophe, C., Jean-Francois, H., Sylvie, J. & Louis, V. Brain. Dev. 30, 169–178 (2008).
Kristien, P., Teodora, C. & Albena, J. Clinical and genetic diversity of SMN1-negative proximal spinal muscular atrophies. Brain 137, 2879–2896 (2014).
Rudnik-Schöneborn, S., Forkert, R., Hahnen, E., Wirth, B. & Zerres, K. Clinical spectrum and diagnostic criteria of infantile spinal muscular atrophy: further delineation on the basis of SMN gene deletion findings. Neuropediatrics 27, 8–15 (1996).
Kumar, P., Henikoff, S. & Ng, P. C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4, 1073–1081 (2009).
Kaindl, A. M., Guenther, U. P., Rudnik-Schöneborn, S., Varon, R., Zerres, K., Schuelke, M. et al. Spinal muscular atrophy with respiratory distress type 1 (SMARD1). J. Child. Neurol. 23, 199–204 (2008).
Grohmann, K., Markus, S., Alexander, D., Katrin, H., Barbara, L., Coleen, A. et al. Mutations in the gene encoding immunoglobulin μ-binding protein 2 cause spinal muscular atrophy with respiratory distress type 1. Nat. Genet. 29, 75–77 (2001).
Guenther, U. P., Handoko, L., Laggerbauer, B., Jablonka, S., Chari, A., Alzheimer, M. et al. IGHMBP2 is a ribosome-associated helicase inactive in the neuromuscular disorder distal SMA type 1 (DSMA1). Hum. Mol. Genet. 18, 1288–1300 (2009).
Ishiura, H., Fukuda, Y., Mitsui, J., Nakahara, Y., Ahsan, B., Takahashi, Y. et al. Posterior column ataxia with retinitis pigmentosa in a Japanese family with a novel mutation in FLVCR1. Neurogenetics 12, 117–121 (2011).
Ishii, A., Saito, Y., Mitsui, J., Ishiura, H., Yoshimura, J., Arai, H. et al. Identification of ATP1A3 mutations by exome sequencing as the cause of alternating hemiplegia of childhood in Japanese patients. PLoS ONE 8, e56120 (2014).
Heng, L. & Richard, D. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Heng, L., Bab, H., Alec, W., Tim, F., Jue, R., Nils, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Shaun, P., Benjamin, N., Kathe, T. B., Lori, T., Manuel, A. R. F., David, B. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 78, 615–628 (2007).
Adzhubei, I. A., Schmidt, S., Peshkin, L., Ramensky, V. E., Gerasimova, A., Bork, P. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
George, D. C., Chakraborty, C., Haneef, S. A., Nagasundaram, N., Chen, L., Zhu, H. et al. Evolution- and structure-based computational strategy reveals the impact of deleterious missense mutations on MODY 2 (maturity-onset diabetes of the young, type 2). Theranostics 4, 366–385 (2014).
Drozdetskiy, A., Cole, C., Procter, J. & Barton, G. J. JPred4: a protein secondary structure prediction server. Nucleic. Acids. Res. 43, 389–394 (2015).
Yang, J. & Zhang, Y. I-TASSER server: new development for protein structure and function predictions. Nucleic. Acids. Res. 43, 174–181 (2015).
Michel, V. J. in Nelson Textbook of Pediatrics 17th edn (eds Richard, E. B., Robert, M. K. & Hal, B. J.2029–2035 (Elsevier, Saunders, Philadelphia, PA, USA, 2001).
Gerald, M. F. in Clinical Pediatric Neurology: A Signs and Symptoms Approach 4th edn (Elsevier, Saunders, Philadelphia, PA, USA, 2001).
JEric, P. G. in Fenichel’s Clinical Pediatric Neurology: A Signs and Symptoms Approach 7th edn (Elsevier, Saunders, Philadelphia, PA, USA, 2013).
Sasaki, M., Sugai, K. & Inagaki, M. in National Center of Neurology and Psychiatry, Department of Child Neurology, A Manual of Diagnosis And Treatment 3rd edn (Shindantochiryousha, Tokyo, Japan, 2015).
Drik, B., Kevin, T. & Martin, R. T. Advances in motor neurone disease. J. R. Soc. Med. 107, 14–21 (2014).
Matthew, P., Henry, H., Jean, J., Quen, M., Brian, H., Mary, R. et al. Severe infantile neuropathy with diaphragmatic weakness and its relationship to SMARD1. Brain 126, 2682–2692 (2003).
Stephen, M. M., Kaashif, A. A., Yalda, M., Erin, L. M., Millan, S. P., David, C. et al. Pontocerebellar hypoplasia: review of classification and genetics, and exclusion of several genes known to be important for cerebellar development. J. Child. Neurol. 26, 288–294 (2011).
Yasmin, N., Peter, Bwee, G. B., Tien, P. T. & Frank, B. Classification, diagnosis and potential mechanisms in pontocerebellar hypoplasia. Orphanet. J. Rare. Dis. 6, 50 (2011).
Okumura, M., Sakuma, C., Miura, M. & Chihara, T. Linking cell surface receptors to microtubules: tubulin folding cofactor D mediates Dscam functions during neuronal morphogenesis. J. Neurosci. 35, 1979–1990 (2015).
Martin, N., Jaubert, J., Gounon, P., Salido, E., Haase, G., Szatanik, M. et al. A missense mutation in TBCE causes progressive motor neuronopathy in mice. Nat. Genet. 32, 443–447 (2002).
Winnie, C., Wim, W., Martin, P., Karoly, S., Jan, W., Edwin, R. et al. Hypoparathyroidism-retardation-dysmorphism syndrome in a girl: a new variant not caused by a TBCE mutation—clinical report and review. Am. J. Med. Genet. 140A, 611–617 (2006).
Don, W. C., Koji, Y. & Pascale, B. Gigaxonin controls vimentin organization through a tubulin chaperone-independent pathway. Hum. Mol. Genet. 18, 1384–1394 (2009).
Hsin-Lan, W., Yuan-Ta, L., Chen-Hung, T., Sue, L. C., Hung, L., Hsiu, M. H. et al. Stathmin, a microtubule-destabilizing protein, is dysregulated in spinal muscular atrophy. Hum. Mol. Genet. 19, 1766–1778 (2010).
Guoling, T., Simi, T. & Nicholas, J. C. Effect of TBCD and its regulatory interactor Arl2 on tubulin and microtubule integrity. Cytoskeleton 67, 706–714 (2010).
Smith, B. N., Ticozzi, N., Fallini, C., Gkazi, A. S., Topp, S., Kenna, K. P. et al. Exome-wide rare variant analysis identifies TUBA4A mutations associated with familial ALS. Neuron 84, 324–331 (2014).
Cleveland, D. W., Yamanaka, K. & Bomont, P. Gigaxonin controls vimentin organization through a tubulin chaperone-independent pathway. Hum. Mol. Genet. 18, 1384–1394 (2009).
Grynberg, M., Jaroszewski, L. & Godzik, A. Domain analysis of the tubulin cofactor system: a model for tubulin folding and dimerization. BMC Bioinformatics 4, 46 (2003).
Groves, M. R. & Barford, D. Topological characteristics of helical repeat proteins. Curr. Opin. Struct. Biol. 9, 383–389 (1999).
National Center for Biotechnology Information. Single Nucleotide Polymorphism Database (dbSNP) (1998) http://www.ncbi.nlm.nih.gov/SNP/. Accessed 22 May 2016.
Anders, L., Lars, L., Jure, P., Goran, L., Poul, N. & Morten, K. Textbook of Structural Biology, (World Scientific, Toh Tuck Link, Singapore, 2009).
Nakayama, M., Iida, M., Koseki, H. & Ohara, O. A gene-targeting approach for functional characterization of KIAA genes encoding extremely large proteins. FASEB J 20, 1718–1720 (2006).
Vihinen, M. Guidelines for reporting and using prediction tools for genetic variation analysis. Hum. Mutat. 7, 248–249 (2010).
Noriko, M., Ryoko, F., Chihiro, O., Takahiro, C., Masayuki, M., Hiroshi, S. et al. Biallelic TBCD mutations cause early-onset neurodegenerative encephalopathy. Am. J. Hum. Genet. 99, 950–961 (2016).
Elisabetta, F., Marcello, N., Serena, C., Isabelle, T., Margaret, G. A., Alessandro, C. et al. Biallelic mutations in TBCD, encoding the tubulin folding cofactor D, perturb microtubule dynamics and cause early-onset encephalopathy. Am. J. Hum. Genet. 99, 962–973 (2016).
Edvardson, S., Tian, G., Cullen, H., Vanyai, H., Ngo, L., Bhat, S. et al. Infantile neurodegenerative disorder associated with mutations in TBCD, an essential gene in the tubulin heterodimer assembly pathway. Hum. Mol. Genet. (e-pub ahead of print 29 August 2016; doi:10.1093/hmg/ddw292 (in press).
National Center for Biotechnology Information. Gene (2003) http://www.ncbi.nlm.nih.gov/gene/. Accessed 12 February 2015.
Wolf, N. I. & van der Knaap, M. S. AGC1 deficiency and cerebral hypomyelination. N. Engl. J. Med. 361, 1997–1998 (2009).
University of California San Francisco Database of Comparative Protein Structure Models (ModBase) (2009) http://modbase.compbio.ucsf.edu/modbase-cgi/index.cgi. Accessed 15 February 2015.
Acknowledgements
We thank the individuals with atypical SMA and their family for their participation in this study. We also thank Drs K Kanako and S Yuko (Department of Neuromuscular Research, National Institute of Neuroscience, National Center of Neurology and Psychiatry) for their helpful comments on the sural nerve biopsy, and Dr K Shiomi (Division of Neurology, Respirology, Endocrinology and Metabolism, Department of Internal Medicine, University of Miyazaki) for help with the nerve conduction study. This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI) for Scientific Research on Innovative Areas (Exploring Molecular Basis for Brain Diseases Based on Personal Genomics), Priority Areas (Applied Genomics), Integrated Database Project and Scientific Research (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a Clinical Research Grant from Miyazaki University Hospital.
Author contributions
TI confirmed the diagnosis in each participating patient, conceived the study, participated in the sequence alignment, designed and performed the experiments, analyzed the data, contributed reagents/materials/analysis tools and drafted the manuscript. AN confirmed the diagnosis for the participating patient designated as case 2, conceived the study, participated in the sequence alignment, designed and performed the experiments, analyzed the data and helped to draft the manuscript. RN confirmed the diagnosis for the participating patient designated as case 1. MU performed the experiments. MO designed and performed the experiments and analyzed the data. HM conceived the study and helped to draft the manuscript. TU conceived and designed the experiments. JM, HI, JY, KD and SM performed the experiments and contributed reagents/materials/analysis tools. NK and NI conceived and designed the experiments. ST conceived and designed the experiments and contributed reagents/materials/analysis tools and drafted manuscript. HN conceived the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Ikeda, T., Nakahara, A., Nagano, R. et al. TBCD may be a causal gene in progressive neurodegenerative encephalopathy with atypical infantile spinal muscular atrophy. J Hum Genet 62, 473–480 (2017). https://doi.org/10.1038/jhg.2016.149
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2016.149
This article is cited by
-
Biallelic pathogenic variants in TBCD-related neurodevelopment disease with mild clinical features
Neurological Sciences (2019)
-
A Faroese founder variant in TBCD causes early onset, progressive encephalopathy with a homogenous clinical course
European Journal of Human Genetics (2018)