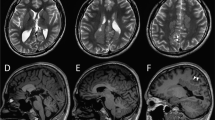
Abstract
Potassium voltage-gated channel subfamily B member 1 (KCNB1) encodes Kv2.1 potassium channel of crucial role in hippocampal neuron excitation homeostasis. KCNB1 mutations are known to cause early-onset infantile epilepsy. To date, 10 KCNB1 mutations have been described in 11 patients. Using whole-exome sequencing, we identified a novel de novo missense (c.1132G>C, p.V378L) KCNB1 mutation in a patient with global developmental delay, intellectual disability, severe speech impairment, but no episode of epilepsy until the lastly examined age of 6 years old. Furthermore, she showed neuropsychiatric symptoms including hyperactivity with irritability, heteroaggressiveness, psychomotor instability and agitation. Our observation might expand the phenotypic spectrum of KCNB1-related phenotypes and raises the issue of the occurrence of the epileptic phenotype.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Villa, C. & Combi, R. Potassium channels and human epileptic phenotypes: an updated overview. Front. Cell Neurosci. 10, 81 (2016).
Ottschytsch, N., Raes, A., Van Hoorick, D. & Snyders, D. J. Obligatory heterotetramerization of three previously uncharacterized Kv channel alpha-subunits identified in the human genome. Proc. Natl Acad. Sci. USA 99, 7986–7991 (2002).
Blaine, J. T. & Ribera, A. B. Heteromultimeric potassium channels formed by members of the Kv2 subfamily. J. Neurosci. Off. J. Soc. Neurosci. 18, 9585–9593 (1998).
Choe, S. Potassium channel structures. Nat. Rev. Neurosci. 3, 115–121 (2002).
Trimmer, J. S. Immunological identification and characterization of a delayed rectifier K+ channel polypeptide in rat brain. Proc. Natl Acad. Sci. USA 88, 10764–10768 (1991).
Speca, D. J., Ogata, G., Mandikian, D., Bishop, H. I., Wiler, S. W., Eum, K. et al. Deletion of the Kv2.1 delayed rectifier potassium channel leads to neuronal and behavioral hyperexcitability. Genes Brain Behav. 13, 394–408 (2014).
Saitsu, H., Akita, T., Tohyama, J., Goldberg-Stern, H., Kobayashi, Y., Cohen, R. et al. De novo KCNB1 mutations in infantile epilepsy inhibit repetitive neuronal firing. Sci. Rep. 5, 15199 (2015).
Srivastava, S., Cohen, J. S., Vernon, H., Barañano, K., McClellan, R., Jamal, L. et al. Clinical whole exome sequencing in child neurology practice. Ann. Neurol. 76, 473–483 (2014).
Allen, N. M., Conroy, J., Shahwan, A., Lynch, B., Correa, R. G., Pena, S. D. et al. Unexplained early onset epileptic encephalopathy: exome screening and phenotype expansion. Epilepsia 57, e12–e17 (2016).
Thiffault, I., Speca, D. J., Austin, D. C., Cobb, M. M., Eum, K. S., Safina, N. P. et al. A novel epileptic encephalopathy mutation in KCNB1 disrupts Kv2.1 ion selectivity, expression, and localization. J. Gen. Physiol. 146, 399–410 (2015).
Torkamani, A., Bersell, K., Jorge, B. S., Bjork, R. L., Friedman, J. R., Bloss, C. S. et al. De novo KCNB1 mutations in epileptic encephalopathy. Ann. Neurol. 76, 529–540 (2014).
Miyake, N., Tsukaguchi, H., Koshimizu, E., Shono, A., Matsunaga, S., Shiina, M. et al. Biallelic mutations in nuclear pore complex subunit NUP107 cause early-childhood-onset steroid-resistant nephrotic syndrome. Am. J. Hum. Genet. 97, 555–566 (2015).
Källberg, M., Wang, H., Wang, S., Peng, J., Wang, Z., Lu, H. et al. Template-based protein structure modeling using the RaptorX web server. Nat. Protoc. 7, 1511–1522 (2012).
de Kovel, C. G. F., Brilstra, E. H., van Kempen, M. J. A., Van’t Slot, R., Nijman, I. J., Afawi, Z . et al. Targeted sequencing of 351 candidate genes for epileptic encephalopathy in a large cohort of patients. Mol Genet Genomic Med. 4, 568–580 (2016).
Acknowledgements
We are grateful to the patient and her family for their participation in the study. This work was supported in part by a grant for Research on Measures for Intractable Diseases, a grant for Comprehensive Research on Disability Health and Welfare, the Strategic Research Program for Brain Science, a grant for Initiative on Rare and Undiagnosed Diseases in Pediatrics from Japan Agency for Medical Research and Development; a grant-in-aid for scientific research on innovative areas (transcription cycle) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT); grants-in-aid for scientific research (B) from the Japan Society for the Promotion of Science; the fund for Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems from the Japan Science and Technology Agency (JST); and the Takeda Science Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Latypova, X., Matsumoto, N., Vinceslas-Muller, C. et al. Novel KCNB1 mutation associated with non-syndromic intellectual disability. J Hum Genet 62, 569–573 (2017). https://doi.org/10.1038/jhg.2016.154
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2016.154
This article is cited by
-
Identifying multi-hit carcinogenic gene combinations: Scaling up a weighted set cover algorithm using compressed binary matrix representation on a GPU
Scientific Reports (2020)
-
Case report: mutation analysis of primary pulmonary lymphoepithelioma-like carcinoma via whole-exome sequencing
Diagnostic Pathology (2019)
-
A familial case of Galloway-Mowat syndrome due to a novel TP53RK mutation: a case report
BMC Medical Genetics (2018)
-
The second point mutation in PREPL: a case report and literature review
Journal of Human Genetics (2018)
-
Monogenic disorders that mimic the phenotype of Rett syndrome
neurogenetics (2018)