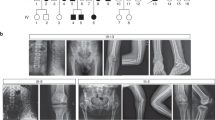
Abstract
Axial spondylometaphyseal dysplasia (axial SMD) is a unique form of SMD characterized by dysplasia of axial skeleton and retinal dystrophy. Recently, C21orf2 has been identified as the first disease gene for axial SMD; however, the presence of genetic heterogeneity is known. In this study, we identified NEK1 as the second disease gene for axial SMD. By whole-exome sequencing in a patient with axial SMD, we identified compound heterozygous mutations of NEK1, c.3107C>G (p.S1036*) and c.3830A>C (p.D1277A), which co-segregated in the family. NEK1 mutations have previously been found in three types of short rib thoracic dystrophy, which have no retinal dystrophy. The skeletal phenotype of our patient was milder than those of previously reported cases with NEK1 mutations and those with axial SMD harboring C21orf2 mutations. Phenotypes associated with NEK1 mutations are variable and the phenotype–genotype corelation in skeletal ciliopathies is challenging.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bonafe, L., Cormier-Daire, V., Hall, C., Lachman, R., Mortier, G., Mundlos, S. et al. Nosology and classification of genetic skeletal disorders: 2015 revision. Am. J. Med. Genet. A 167A, 2869–2892 (2015).
Ehara, S., Kim, OH., Maisawa, S., Takasago, Y. & Nishimura, G. Axial spondylometaphyseal dysplasia. Eur. J. Pediatr. 156, 627–630 (1997).
Isidor, B., Baron, S., Khau van Kien, P., Bertrand, A. M., David, A. et al. Axial spondylometaphyseal dysplasia: confirmation and further delineation of a new SMD with retinal dystrophy. Am. J. Med. Genet. A 152A, 1550–1554 (2010).
Suzuki, S., Kim, O. H., Makita, Y., Saito, T., Lim, G. Y., Cho, T. J. et al. Axial spondylometaphyseal dysplasia: additional reports. Am. J. Med. Genet. A 155A, 2521–2528 (2011).
Wang, Z., Iida, A., Miyake, N., Nishiguchi, KM., Fujita, K., Nakazawa, T. et al. Axial spondylometaphyseal dysplasia is caused by C21orf2 mutations. PLoS ONE 11, e0150555 (2016).
Guo, L., Girisha, K. M., Iida, A., Hebbar, M., Shukla, A., Shah, H. et al. Identification of a novel LRRK1 mutation in a family with osteosclerotic metaphyseal dysplasia. J. Hum. Genet. (e-pub ahead of print 10 November 2016; doi:10.1038/jhg.2016.136).
Kumar, P., Henikoff, S. & Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4, 1073–1081 (2009).
Adzhubei, I. A., Schmidt, S., Peshkin, L., Ramensky, V. E., Gerasimova, A., Bork, P. et al A method and server for predicting damaging missense mutations. Nat. Methods. 7, 248–249 (2010).
Schwarz, J. M., Cooper, D. N., Schuelke, M. & Seelow, D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat. Methods 11, 361–362 (2014).
Fry, A. M., O’Regan, L., Sabir, S. R. & Bayliss, R. Cell cycle regulation by the NEK family of protein kinases. J. Cell Sci. 125, 4423–4433 (2012).
Kim, G., Kim, J. Y. & Choi, H. S. Peptidyl–prolyl cis/trans isomerase NIMA-Interacting 1 as a therapeutic target in hepatocellular carcinoma. Biol. Pharm. Bull. 38, 975–979 (2015).
Wheway, G., Schmidts, M., Mans, D. A., Szymanska, K., Nguyen, T. M., Racher, H. et al. An siRNA-based functional genomics screen for the identification of regulators of ciliogenesis and ciliopathy genes. Nat. Cell Biol. 17, 1074–1087 (2015).
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H. et al. Clustal W and Clustal X version 2.0. Bioinformatics. 23, 2947–2948 (2007).
Thiel, C., Kessler, K., Giessl, A., Dimmler, A., Shalev, S. A., Beinder, E. et al. NEK1 mutations cause short-rib polydactyly syndrome type Majewski. Am. J. Hum. Genet. 88, 106–114 (2011).
Chen, C. P., Chang, T. Y., Chen, C. Y., Wang, T. Y., Tsai, F. J., Wu, P. C. et al. Short rib-polydactyly syndrome type II (Majewski): prenatal diagnosis, perinatal imaging findings and molecular analysis of the NEK1 gene. Taiwan J. Obstet. Gynecol 51, 100–105 (2012).
El Hokayem, J., Huber, C., Couvé, A., Aziza, J., Baujat, G., Bouvier, R. et al. NEK1 and DYNC2H1 are both involved in short rib polydactyly Majewski type but not in Beemer Langer cases. J. Med. Genet. 49, 227–233 (2012).
McInerney-Leo, A. M., Harris, J. E., Leo, P. J., Marshall, M. S., Gardiner, B., Kinning, E. et al. Whole exome sequencing is an efficient, sensitive and specific method for determining the genetic cause of short-rib thoracic dystrophies. Clin. Genet. 88, 550–557 (2015).
Vogler, C., Homan, S., Pung, A., Thorpe, C., Barker, J., Birkenmeier, E. H. et al. Clinical and pathologic findings in two new allelic murine models of polycystic kidney disease. J. Am. Soc. Nephrol. 10, 2534–2539 (1999).
Huber, C. & Cormier-Daire, V. Ciliary disorder of the skeleton. Am. J. Med. Genet. C 160C, 165–174 (2012).
Geister, K. A. & Camper, S. A. Advances in skeletal dysplasia genetics. Annu. Rev. Genomics Hum. Genet 16, 199–227 (2015).
Chen, C. P., Chern, S. R., Chang, T. Y., Su, Y. N., Chen, Y. Y., Su, J. W. et al. Prenatal diagnosis and molecular genetic analysis of short rib-polydactyly syndrome type III (Verma-Naumoff) in a second-trimester fetus with a homozygous splice site mutation in intron 4 in the NEK1 gene. Taiwan J. Obstet. Gynecol. 51, 266–270 (2012).
Upadhya, P., Birkenmeier, E. H., Birkenmeier, C. S. & Barker, J. E. Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc. Natl. Acad. Sci. U S A. 97, 217–221 (2000).
Acknowledgements
We thank the family for participating in the study. This study is supported by KAKENHI Grant-in-Aid for Scientific Research (B) (NMi, No 25293235), Takeda Science Foundation (ZW), and research grants from Japan Agency For Medical Research and Development (AMED) (SI, NMa, No 16ek0109068h0003). This study is also supported by the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet and by grants from Kronprinsessan Lovisas, Stiftelsen Frimurare Barnhuset in Stockholm, Hjärnfonden, Axel Tielmans Minnesfond, Samariten and Promobilia Foundations (GG, AN).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Wang, Z., Horemuzova, E., Iida, A. et al. Axial spondylometaphyseal dysplasia is also caused by NEK1 mutations. J Hum Genet 62, 503–506 (2017). https://doi.org/10.1038/jhg.2016.157
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2016.157
This article is cited by
-
Exploring NEK1 genetic variability in Italian amyotrophic lateral sclerosis patients
Journal of Neurology (2025)
-
Variants in CFAP410 cause a range of retinal and skeletal phenotypes
npj Genomic Medicine (2025)
-
Heterozygous mutations in the straitjacket region of the latency-associated peptide domain of TGFB2 cause Camurati–Engelmann disease type II
Journal of Human Genetics (2024)
-
Recent Updates on the Genetics of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia
Molecular Neurobiology (2022)
-
Skeletal ciliopathies: a pattern recognition approach
Japanese Journal of Radiology (2020)