Abstract
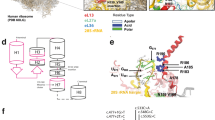
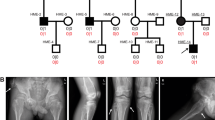
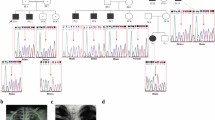
Spondylo-epi-metaphyseal dysplasia (SEMD) is a group of inherited skeletal diseases characterized by the anomalies in spine, epiphyses and metaphyses. SEMD is highly heterogeneous and >20 distinct entities have been identified. Here we describe a novel type of SEMD in two unrelated Turkish patients who presented with severe platyspondyly, kyphoscoliosis, pelvic distortion, constriction of the proximal femora and brachydactyly. Although these phenotypes overlap considerably with some known SEMDs, they had a novel causal gene, exostosin-like glycosyltransferase 3 (EXTL3), that encodes a glycosyltransferase involved in the synthesis of heparin and heparan sulfate. The EXTL3 mutation identified in the patients was a homozygous missense mutation (c.953C>T) that caused a substitution in a highly conserved amino acid (p.P318L). The enzyme activity of the mutant EXTL3 protein was significantly decreased compared to the wild-type protein. Both patients had spinal cord compression at the cranio-vertebral junction and multiple liver cysts since early infancy. One of the patients showed severe immunodeficiency, which is considered non-fortuitous association. Our findings would help define a novel type of SEMD caused by EXTL3 mutations.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Guo, L ., Elcioglu, N. H ., Iida, A ., Demirkol, Y. K ., Aras, S ., Matsumoto, N. et al. Novel and recurrent XYLT1 mutations in two Turkish families with Desbuquois dysplasia, type 2. J. Hum. Genet. 62, 447–451 (2016).
Guo, L ., Girisha, K. M ., Iida, A ., Hebbar, M ., Shukla, A ., Shah, H. et al. Identification of a novel LRRK1 mutation in a family with osteosclerotic metaphyseal dysplasia. J. Hum. Genet. 62, 437–441 (2016).
Wang, Z ., Horemuzova, E ., Iida, A ., Guo, L ., Liu, Y ., Matsumoto, N. et al. Axial spondylometaphyseal dysplasia is also caused by NEK1 mutations. J. Hum. Genet. (e-pub ahead of print 26 January 2017; doi: 10.1038/jhg.2016.157).
Miyatake, S ., Tada, H ., Moriya, S ., Takanashi, J ., Hirano, Y ., Hayashi, M. et al. Atypical giant axonal neuropathy arising from a homozygous mutation by uniparental isodisomy. Clin. Genet. 87, 395–397 (2015).
Mizumoto, S ., Ikegawa, S. & Sugahara, K. Human genetic disorders caused by mutations in genes encoding biosynthetic enzymes for sulfated glycosaminoglycans. J. Biol. Chem. 288, 10953–10961 (2013).
Mizumoto, S ., Yamada, S. & Sugahara, K. Human genetic disorders and knockout mice deficient in glycosaminoglycan. Biomed. Res. Int. 2014, 495764 (2014).
Baasanjav, S ., Al-Gazali, L ., Hashiguchi, T ., Mizumoto, S ., Fischer, B ., Horn, D. et al. Faulty initiation of proteoglycan synthesis causes cardiac and joint defects. Am. J. Hum. Genet. 89, 15–27 (2011).
Kim, B. T ., Kitagawa, H ., Tamura, J ., Saito, T ., Kusche-Gullberg, M ., Lindahl, U. et al. Human tumor suppressor EXT gene family members EXTL1 and EXTL3 encode alpha 1,4-N-acetylglucosaminyltransferases that likely are involved in heparan sulfate/heparin biosynthesis. Proc. Natl Acad. Sci. USA 109, 7176–7181 (2001).
Kitagawa, H ., Tsuchida, K ., Ujikawa, M. & Sugahara, K. Detection and characterization of UDP-GalNAc: chondroitin N-acetylgalactosaminyltransferase in bovine serum using a simple assay method. J. Biochem. 117, 1083–1087 (1995).
Bonafe, L ., Cormier-Daire, V ., Hall, C ., Lachman, R ., Mortier, G ., Mundlos, S. et al. Nosology and classification of genetic skeletal disorders: 2015 revision. Am. J. Med. Genet. A 167, 2869–2892 (2015).
Moosa, S ., Fano, V ., Obregon, M. G ., Altmüller, J ., Thiele, H ., Nürnberg, P. et al. A novel homozygous PAM16 mutation in a patient with a milder phenotype and longer survival. Am. J. Med. Genet. A 170, 2436–2439 (2016).
Lee, H ., Nevarez, L ., Lachman, R. S ., Wilcox, W. R ., Krakow, D ., Cohn, D. H. et al. A second locus for Schneckenbecken dysplasia identified by a mutation in the gene encoding inositol polyphosphate phosphatase-like 1 (INPPL1). Am. J. Med. Genet. A 167A, 2470–2473 (2015).
Takahashi, I ., Noguchi, N ., Nata, K ., Yamada, S ., Kaneiwa, T ., Mizumoto, S. et al. Important role of heparan sulfate in postnatal islet growth and insulin secretion. Biochem. Biophys. Res. Commun. 383, 113–118 (2009).
Holmborn, K ., Habicher, J ., Kasza, Z ., Eriksson, A. S ., Filipek-Gorniok, B ., Gopal, S. et al. On the roles and regulation of chondroitin sulfate and heparan sulfate in zebrafish pharyngeal cartilage morphogenesis. J. Biol. Chem. 287, 33905–33916 (2012).
Norton, W. H. J ., Ledin, J ., Grandel, H. & Neumann, C. J. HSPG synthesis by zebrafish Ext2 and Extl3 is required for Fgf10 signalling during limb development. Development 132, 4963–4973 (2005).
Yamada, Y ., Arikawa-Hirasawa, E ., Watanabe, H ., Takami, H. & Hassell, J. R. Perlecan is essential for cartilage and cephalic development. Nat. Genet. 23, 354–358 (1999).
Viviano, B. L ., Silverstein, L ., Pflederer, C ., Paine-Saunders, S ., Mills, K. & Saunders, S. Altered hematopoiesis in glypican-3-deficient mice results in decreased osteoclast differentiation and a delay in endochondral ossification. Dev. Biol. 282, 152–162 (2005).
Häcker, U ., Nybakken, K. & Perrimon, N. Heparan sulphate proteoglycans: the sweet side of development. Nat. Rev. Mol. Cell Biol. 6, 530–541 (2005).
Fuerer, C ., Habib, S. J. & Nusse, R. A study on the interactions between heparan sulfate proteoglycans and Wnt proteins. Dev. Dyn. 239, 184–190 (2010).
Kikuchi, A ., Yamamoto, H ., Sato, A. & Matsumoto, S. New insights into the mechanism of Wnt signaling pathway activation. Int. Rev. Cell Mol. Biol. 291, 21–71 (2011).
Olwin, B. B. & Rapraeger, A. Repression of myogenic differentiation by aFGF, bFGF, and K-FGF is dependent on cellular heparan sulfate. J. Cell Biol. 118, 631–639 (1992).
Rapraeger, A. C ., Krufka, A. & Olwin, B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science 252, 1705–1708 (1991).
Shimokawa, K ., Kimura-Yoshida, C ., Nagai, N ., Mukai, K ., Matsubara, K ., Watanabe, H. et al. Cell surface heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Dev. Cell 21, 257–272 (2011).
Manton, K. J ., Leong, D. F. M ., Cool, S. M. & Nurcombe, V. Disruption of heparan and chondroitin sulfate signaling enhances mesenchymal stem cell-derived osteogenic differentiation via bone morphogenetic protein signaling pathways. Stem Cells 25, 2845–2854 (2007).
Lilic, D. New perspectives on the immunology of chronic mucocutaneous candidiasis. Curr. Opin. Infect. Dis. 15, 143–147 (2002).
Warrier, S. A. & Sathasivasubramanian, S. Human immunodeficiency virus induced oral candidiasis. J. Pharm. Bioallied Sci. 7, 812 (2015).
Pichard, D. C ., Freeman, A. F. & Cowen, E. W. Primary immunodeficiency update: Part II. Syndromes associated with mucocutaneous candidiasis and noninfectious cutaneous manifestations. J. Am. Acad. Dermatol. 73, 367–381 (2015).
Volpi, S ., Yamazaki, Y ., Brauer, P. M ., van Rooijen, E ., Hayashida, A ., Slavotinek, A. et al. EXTL3 mutations cause skeletal dysplasia, immune deficiency, and developmental delay. J. Exp. Med. 214, 623–637 (2017).
Oud, M. M ., Tuijnenburg, P ., Hempel, M ., van Vlies, N ., Ren, Z ., Ferdinandusse, S. et al. Mutations in EXTL3 cause neuro-immuno-skeletal dysplasia syndrome. Am. J. Hum. Genet. 100, 281–296 (2017).
Acknowledgements
We thank the patients and their families for their help to the study. This study was supported in part by research grants from Japan Agency For Medical Research and Development (AMED; contract No 14525125), by a Grant-in-Aid for Scientific Research (C) 16K08251 (to SM) from the Japan Society for the Promotion of Science, Japan, and by the Nakatomi Foundation (to SM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Guo, L., Elcioglu, N., Mizumoto, S. et al. Identification of biallelic EXTL3 mutations in a novel type of spondylo-epi-metaphyseal dysplasia. J Hum Genet 62, 797–801 (2017). https://doi.org/10.1038/jhg.2017.38
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jhg.2017.38
This article is cited by
-
An ultra-rare case of immunoskeletal dysplasia with neurodevelopmental abnormalities in an Indian patient with homozygous c.953C > T variant in EXTL3 gene: a case report
BMC Pediatrics (2022)
-
The structure of EXTL3 helps to explain the different roles of bi-domain exostosins in heparan sulfate synthesis
Nature Communications (2022)
-
Inborn errors of thymic stromal cell development and function
Seminars in Immunopathology (2021)
-
Specific functions of Exostosin-like 3 (EXTL3) gene products
Cellular & Molecular Biology Letters (2020)
-
Dysosteosclerosis is also caused by TNFRSF11A mutation
Journal of Human Genetics (2018)