Abstract
Objective:
To examine the effect of sildenafil therapy on development of severe retinopathy of prematurity (ROP) requiring surgical intervention in premature infants.
Study Design:
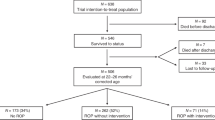
We identified premature infants who were discharged from Pediatrix Medical Group neonatal intensive care units from 2003 to 2012 and who received an ophthalmologic exam. We matched each infant exposed to sildenafil before first eye exam to three nonexposed infants using propensity scoring to control for differences in baseline infant characteristics. We evaluated the association between sildenafil exposure and development of severe ROP using conditional logistic regression.
Result:
Of the 57 815 infants meeting inclusion criteria, 88 were exposed to sildenafil. We matched 81/88 (92%) sildenafil-exposed with 243 nonexposed infants. There was no difference in the proportion of infants who developed severe ROP in the sildenafil-exposed vs nonexposed groups (17/81 (21%) vs 38/243 (16%), P=0.27). On adjusted analysis, there was no difference in severe ROP in the sildenafil-exposed vs nonexposed infants (odds ratio=1.46, 95% confidence interval=0.76 to 2.82, P=0.26).
Conclusion:
We did not observe an association between risk of severe ROP and sildenafil exposure before first eye exam in this cohort of premature infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Malik M, Nagpal R . Emerging role of sildenafil in neonatology. Indian Pediatr 2011; 48 (1): 11–13.
Samiee-Zafarghandy S, Smith PB, van den Anker J . Safety of sildenafil in infants. Pediatr Crit Care Med 2014; 15 (4): 362–368.
Cordell W . Retinal effects of 6 months of daily use of tadalafil or sildenafil. Arch Ophthalmol 2009; 127 (4): 367.
Azzouni F, Abu Samra K . Are phosphodiesterase type 5 inhibitors associated with vision-threatening adverse events? A critical analysis and review of the literature. J Sex Med 2011; 8 (10): 2894–2903.
Marsh C . Severe retinopathy of prematurity (ROP) in a premature baby treated with sildenafil acetate (Viagra) for pulmonary hypertension. Br J Ophthalmol 2004; 88 (2): 306–307.
Hsieh E, Hornik C, Clark R, Laughon M, Benjamin D, Smith PB . Medication use in the neonatal intensive care unit. Am J Perinatol 2013; 31 (9): 811–822.
Olsen I, Groveman S, Lawson M, Clark R, Zemel B . New intrauterine growth curves based on United States data. Pediatrics 2010; 125 (2): e214–e224.
Rubin DB, Thomas N . Matching using estimated propensity scores: relating theory to practice. Biometrics 1996; 52 (1): 249–264.
Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T . Variable selection for propensity score models. Am J Epidemiol 2006; 163 (12): 1149–1156.
Leuven E, Sianesi B . Psmatch2: stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Available from http://ideas.repec.org/c/boc/bocode/s432001.html Created 2003; revised 2015.
Fang A, Guy K, König K . The effect of sildenafil on retinopathy of prematurity in very preterm infants. J Perinatol 2012; 33 (3): 218–221.
Kehat R, Bonsall D, North R, Connors B . Ocular findings of oral sildenafil use in term and near-term neonates. J AAPOS 2010; 14 (2): 159–162.
Laties A . Vision disorders and phosphodiesterase type 5 inhibitors. Drug Saf 2009; 32 (1): 1–18.
Center for Drug Evaluation and Research NDA 020895/S-21 Viagra (Sildenafil Citrate) Tablets: Clinical Pharmacology/Biopharmaceutics Review. Department of Health and Human Services, US Food and Drug Administration: Rockville, MD, 1998.
Center for Drug Evaluation and Research Study 148–223: a double-blind, randomized, placebo-controlled, four-period crossover study to assess the effect of orally administered sildenafil (50, 100, and 200 mg) on visual function in healthy male volunteers. In: Viagra (Sildenafil): Joint Clinical Review for NDA-20-895. Center for Drug Evaluation and Research, FDA: Washington, DC, 1998, pp 160–161.
Donahue S, Taylor R . Pupil-sparing third nerve palsy associated with sildenafil citrate (Viagra). Am J Ophthalmol 1998; 126 (3): 476–477.
Egan R, Pomeranz H . Sildenafil (Viagra) associated anterior ischemic optic neuropathy. Arch Ophthalmol 2000; 118 (2): 291–292.
Cunningham A, Smith K . Anterior ischemic optic neuropathy associated with Viagra. J Neuroophthalmol 2001; 21 (1): 22–25.
Foresta C, Caretta N, Zuccarello D, Poletti A, Biagioli A, Caretti L et al. Expression of the PDE5 enzyme on human retinal tissue: new aspects of PDE5 inhibitors ocular side effects. Eye 2007; 22 (1): 144–149.
Gerometta R, Alvarez L, Candia O . Effect of sildenafil citrate on intraocular pressure and blood pressure in human volunteers. Exp Eye Res 2011; 93 (1): 103–107.
Harris A, Kagemann L, Ehrlich R, Ehrlich Y, Lopez C, Purvin V . The effect of sildenafil on ocular blood flow. Br J Ophthalmol 2008; 92 (4): 469–473.
Vance S, Imamura Y, Freund K . The effects of sildenafil citrate on choroidal thickness as determined by enhanced depth imaging optical coherence tomography. Retina 2011; 31 (2): 332–335.
Cavallaro G, Filippi L, Bagnoli P, La Marca G, Cristofori G, Raffaeli G et al. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol 2013; 92 (1): 2–20.
Heywood R, Osterloh IH, Phillips SC . Sildenafil causes a dose- and time-dependent downregulation of phosphodiesterase type 6 expression in the rat retina. Int J Impot Res 2000; 12 (4): 241–244.
Jackson G, Benjamin N, Jackson N, Allen MJ . Effects of sildenafil citrate on human hemodynamics. Am J Cardiol 1999; 83 (5A): 13C–20C.
International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005; 123 (7): 991–999.
Misra A, Heckford E, Curley A, Allen L . Do current retinopathy of prematurity screening guidelines miss the early development of pre-threshold type 1 ROP in small for gestational age neonates? Eye 2007; 22 (6): 825–829.
Acknowledgements
This work was performed under the Best Pharmaceuticals for Children Act – Pediatric Trials Network (Government Contract HHSN275201000003I).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
Dr Smith receives salary support for research from the National Institutes of Health (NIH) and the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (HHSN275201000003I and 1R01-HD081044-01) and the Food and Drug Administration (1R18-FD005292-01); he also receives research support from Cempra Pharmaceuticals (subaward to HHS0100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr van den Anker receives salary support for research from the NIH (5K24DA027992, 5U54HD071601, 5R01HD060543). Dr Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117). Dr Laughon receives support from the US government for his work in pediatric and neonatal clinical pharmacology (HHSN267200700051C, PI: Benjamin, under the Best Pharmaceuticals for Children Act) and from the NICHD (5K23HD068497-01). The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website
Supplementary information
Rights and permissions
About this article
Cite this article
Samiee-Zafarghandy, S., van den Anker, J., Laughon, M. et al. Sildenafil and retinopathy of prematurity risk in very low birth weight infants. J Perinatol 36, 137–140 (2016). https://doi.org/10.1038/jp.2015.126
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/jp.2015.126
This article is cited by
-
Early diagnosis and targeted approaches to pulmonary vascular disease in bronchopulmonary dysplasia
Pediatric Research (2022)
-
Prophylactic Sildenafil in Preterm Infants at Risk of Bronchopulmonary Dysplasia: A Pilot Randomized, Double-Blinded, Placebo-Controlled Trial
Clinical Drug Investigation (2019)
-
Controversies in the identification and management of acute pulmonary hypertension in preterm neonates
Pediatric Research (2017)
-
More safety data: what about efficacy of sildenafil?
Journal of Perinatology (2016)