Abstract
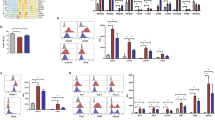
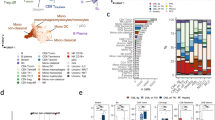
Dasatinib treatment markedly increases the number of large granular lymphocytes (LGLs) in a proportion of Ph+ leukemia patients, which associates with a better prognosis. The lymphocytosis is predominantly observed in cytomegalovirus (CMV)-seropositive patients, yet detectable CMV reactivation exists only in a small fraction of patients. Thus, etiology of the lymphocytosis still remains unclear. Here, we identified NK cells as the dominant LGLs expanding in dasatinib-treated patients, and applied principal component analysis (PCA) to an extensive panel of NK cell markers to explore underlying factors in NK cell activation. PCA displayed phenotypic divergence of NK cells that reflects CMV-associated differentiation and genetic differences, and the divergence was markedly augmented in CMV-seropositive dasatinib-treated patients. Notably, the CMV-associated highly differentiated status of NK cells was already observed at leukemia diagnosis, and was further enhanced after starting dasatinib in virtually all CMV-seropositive patients. Thus, the extensive characterization of NK cells by PCA strongly suggests that CMV is an essential factor in the NK cell activation, which progresses stepwise during leukemia and subsequent dasatinib treatment most likely by subclinical CMV reactivation. This study provides a rationale for the exploitation of CMV-associated NK cell activation for treatment of leukemias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kim DH, Kamel-Reid S, Chang H, Sutherland R, Jung CW, Kim HJ et al. Natural killer or natural killer/T cell lineage large granular lymphocytosis associated with dasatinib therapy for Philadelphia chromosome positive leukemia. Haematologica 2009; 94: 135–139.
Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia 2009; 23: 1398–1405.
Kreutzman A, Juvonen V, Kairisto V, Ekblom M, Stenke L, Seggewiss R et al. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood 2010; 116: 772–782.
Li J, Rix U, Fang B, Bai Y, Edwards A, Colinge J et al. A chemical and phosphoproteomic characterization of dasatinib action in lung cancer. Nat Chem Biol 2010; 6: 291–299.
Weichsel R, Dix C, Wooldridge L, Clement M, Fenton-May A, Sewell AK et al. Profound inhibition of antigen-specific T-cell effector functions by dasatinib. Clin Cancer Res 2008; 14: 2484–2491.
Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R et al. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood 2008; 111: 1366–1377.
Blake SJ, Bruce Lyons A, Fraser CK, Hayball JD, Hughes TP . Dasatinib suppresses in vitro natural killer cell cytotoxicity. Blood 2008; 111: 4415–4416.
Fujita H, Kitawaki T, Sato T, Maeda T, Kamihira S, Takaori-Kondo A et al. The tyrosine kinase inhibitor dasatinib suppresses cytokine production by plasmacytoid dendritic cells by targeting endosomal transport of CpG DNA. Eur J Immunol 2013; 43: 93–103.
Kreutzman A, Ladell K, Koechel C, Gostick E, Ekblom M, Stenke L et al. Expansion of highly differentiated CD8+ T-cells or NK-cells in patients treated with dasatinib is associated with cytomegalovirus reactivation. Leukemia 2011; 25: 1587–1597.
Tanaka H, Nakashima S, Usuda M . Rapid and sustained increase of large granular lymphocytes and rare cytomegalovirus reactivation during dasatinib treatment in chronic myelogenous leukemia patients. Int J Hematol 2012; 96: 308–319.
Vivier E, Anfossi N . Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat Rev Immunol 2004; 4: 190–198.
Ringner M . What is principal component analysis? Nat Biotechnol 2008; 26: 303–304.
Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM . Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity 2012; 36: 142–152.
Bezman NA, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y et al. Molecular definition of the identity and activation of natural killer cells. Nat Immunol 2012; 13: 1000–1009.
Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med 2013; 5: 208ra145.
Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M . Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004; 104: 3664–3671.
Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci USA 2011; 108: 14725–14732.
Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012; 119: 2665–2674.
Muntasell A, Vilches C, Angulo A, Lopez-Botet M . Adaptive reconfiguration of the human NK-cell compartment in response to cytomegalovirus: a different perspective of the host-pathogen interaction. Eur J Immunol 2013; 43: 1133–1141.
Lopez-Botet M, Muntasell A, Vilches C . The CD94/NKG2C+ NK-cell subset on the edge of innate and adaptive immunity to human cytomegalovirus infection. Semin Immunol 2014; 26: 145–151.
Miyashita R, Tsuchiya N, Hikami K, Kuroki K, Fukazawa T, Bijl M et al. Molecular genetic analyses of human NKG2C (KLRC2) gene deletion. Int Immunol 2004; 16: 163–168.
Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006; 25: 331–342.
Beziat V, Liu LL, Malmberg JA, Ivarsson MA, Sohlberg E, Bjorklund AT et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood 2013; 121: 2678–2688.
Djaoud Z, David G, Bressollette C, Willem C, Rettman P, Gagne K et al. Amplified NKG2C+ NK cells in cytomegalovirus (CMV) infection preferentially express killer cell Ig-like receptor 2DL: functional impact in controlling CMV-infected dendritic cells. J Immunol 2013; 191: 2708–2716.
van Stijn A, Rowshani AT, Yong SL, Baas F, Roosnek E, ten Berge IJ et al. Human cytomegalovirus infection induces a rapid and sustained change in the expression of NK cell receptors on CD8+ T cells. J Immunol 2008; 180: 4550–4560.
Bjorkstrom NK, Beziat V, Cichocki F, Liu LL, Levine J, Larsson S et al. CD8 T cells express randomly selected KIRs with distinct specificities compared with NK cells. Blood 2012; 120: 3455–3465.
Beziat V, Dalgard O, Asselah T, Halfon P, Bedossa P, Boudifa A et al. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur J Immunol 2012; 42: 447–457.
Imagawa J, Tanaka H, Matsumoto K, Morita K, Harada Y, Harada H . A sharp fluctuation in peripheral blood cells shortly after dasatinib administration. Int J Hematol 2012; 96: 194–199.
Mustjoki S, Auvinen K, Kreutzman A, Rousselot P, Hernesniemi S, Melo T et al. Rapid mobilization of cytotoxic lymphocytes induced by dasatinib therapy. Leukemia 2013; 27: 914–924.
Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med 2011; 208: 13–21.
Petitdemange C, Becquart P, Wauquier N, Beziat V, Debre P, Leroy EM et al. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog 2011; 7: e1002268.
Lee KC, Ouwehand I, Giannini AL, Thomas NS, Dibb NJ, Bijlmakers MJ . Lck is a key target of imatinib and dasatinib in T-cell activation. Leukemia 2010; 24: 896–900.
Salih J, Hilpert J, Placke T, Grunebach F, Steinle A, Salih HR et al. The BCR/ABL-inhibitors imatinib, nilotinib and dasatinib differentially affect NK cell reactivity. Int J Cancer 2010; 127: 2119–2128.
Hermouet S, Sutton CA, Rose TM, Greenblatt RJ, Corre I, Garand R et al. Qualitative and quantitative analysis of human herpesviruses in chronic and acute B cell lymphocytic leukemia and in multiple myeloma. Leukemia 2003; 17: 185–195.
Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol 2012; 30: 232–238.
Rolle A, Brodin P . Immune adaptation to environmental influence: the case of NK Cells and HCMV. Trends Immunol 2016; 37: 233–243.
Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015; 42: 431–442.
Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015; 42: 443–456.
Mahon F-X, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 2010; 11: 1029–1035.
Imagawa J, Tanaka H, Okada M, Nakamae H, Hino M, Murai K et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol 2015; 2: e528–e535.
Mizoguchi I, Yoshimoto T, Katagiri S, Mizuguchi J, Tauchi T, Kimura Y et al. Sustained upregulation of effector natural killer cells in chronic myeloid leukemia after discontinuation of imatinib. Cancer Sci 2013; 104: 1146–1153.
Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Nuebling T, Raum T et al. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D-mediated NK cell responses. J Immunol 2012; 189: 1360–1371.
Moretta A, Marcenaro E, Sivori S, Della Chiesa M, Vitale M, Moretta L . Early liaisons between cells of the innate immune system in inflamed peripheral tissues. Trends Immunol 2005; 26: 668–675.
Duerkop BA, Hooper LV . Resident viruses and their interactions with the immune system. Nat Immunol 2013; 14: 654–659.
Acknowledgements
We thank K Ohmori, K Fukunaga, M Tozaki, M Takahara and M Ohnishi for the excellent technical support. This work was supported by research funding from Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Ishiyama, Ki., Kitawaki, T., Sugimoto, N. et al. Principal component analysis uncovers cytomegalovirus-associated NK cell activation in Ph+ leukemia patients treated with dasatinib. Leukemia 31, 203–212 (2017). https://doi.org/10.1038/leu.2016.174
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/leu.2016.174