Abstract
We present a direct-write patterning method for the realization of electroluminescent (EL) line art using a surface-emissive light-emitting electrochemical cell with its electrolyte and EL material separated into a bilayer structure. The line-art emission is achieved through subtractive patterning of the electrolyte layer with a stylus, and the single-step patterning can be either manual for personalization and uniqueness or automated for high throughput and repeatability. We demonstrate that the light emission is effectuated by cation-assisted electron injection in the patterned regions and that the resulting emissive lines can be as narrow as a few micrometers. The versatility of the method is demonstrated through the attainment of a wide range of light-emission patterns and colors using a variety of different materials. We propose that this low-voltage-driven and easy-to-modify luminescent line-art technology could be of interest for emerging applications, such as active packaging and personalized gadgets.
Similar content being viewed by others
Introduction
The concept of a low-cost, thin, and lightweight device that can turn personalized sketches and handwriting to life through light emission is intriguing, and it also fills a distinct consumer need with the advent of active packaging and personalized gadgets, among other technologies1,2. However, to date, no technology on the market appears to provide an ideal fit for the requirements of these emerging applications, and innovative reports on functional patterned emission are rather scarce3,4. The few available examples in the public domain include photo-charged phosphorescent paints that feature a relatively low brightness and short glow time5,6,7,8, masked or electrode-patterned surface-emissive devices that commonly suffer from poorly scalable fabrication9, inefficient operation, or limited emissive shapes10,11,12, and passive or active-matrix-driven displays that are complex and relatively costly devices that depend on a software input for personalization13,14.
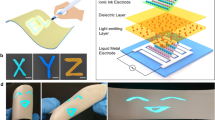
Here, we introduce subtractive stylus patterning of a light-emitting electrochemical cell (LEC), with its constituent electrolyte and electroluminescent (EL) compound separated into a bilayer structure, as a straightforward means for the realization of a luminescent handwritten image. The key steps in the device fabrication process are schematically presented in Figure 1. In brief, the electrolyte layer is deposited from solution on top of a transparent electrode, which is in turn positioned on a transparent substrate (Figure 1a). The electrolyte layer is thereafter patterned through the contact motion of (or ‘writing’ with) a sharp stylus (Figure 1b). The EL compound is subsequently solution-deposited on top of the patterned electrolyte to form the bilayer structure (Figure 1c). The device structure is finally completed by the deposition of a reflective top electrode (Figure 1d).
Fabrication of a bilayer light-emitting electrochemical cell featuring line-art light emission. For clarity, the dimensions are not to scale. (a) The electrolyte is deposited from solution on top of a transparent electrode. (b) The electrolyte layer is mechanically patterned into the desired emission pattern using a stylus. (c) The electroluminescent (EL) material is deposited from solution to complete the bilayer structure. (d) The complete device, following drying of the bilayer sandwich and deposition of a reflective top electrode.
The light-emission pattern is effectively defined by the selected motion of the stylus, and we show that the emission lines can feature a high resolution on the order of a few micrometers. Therefore, the method also allows for personal touch, where the stylus motion is comparable to the manual writing with a pen. Conventional LEC devices have recently been fabricated with high-yield solution-based fabrication methods under ambient air, and the technology thereby qualifies as being truly cost-efficient15,16,17. Finally, we report that a wide range of different EL compounds and electrolytes are compatible with the patterning method, and we demonstrate that essentially any light-emission color and pattern is attainable from a line-art bilayer LEC driven by a few volts.
Materials and methods
We have investigated a wide variety of different materials, and one representative example of the device fabrication procedure is described below. More details on device fabrication and characterization, as well as information on the other investigated material systems, are included in the Supplementary Information.
The electrolyte ink was prepared by separately dissolving the ion-transport material poly(ethylene oxide) (PEO, MW = 5 × 106 g mol−1) and the salt KCF3SO3 in cyclohexanone at a concentration of 10 g l−1 and thereafter mixing the two solutions in a {PEO:KCF3SO3} = {4:1} mass ratio. The EL ink was prepared by dissolving the yellow-emitting conjugated polymer Super Yellow (Livilux PDY-132, Merck, Germany) in toluene at a concentration of 7 g l−1. The electrolyte ink was spin-coated on a carefully cleaned indium-tin-oxide (ITO)-coated glass substrate such that a 160-nm-thick electrolyte layer formed. The electrolyte layer was then patterned with either a syringe needle or a mechanized pen with the lead replaced by a sharp stylus. The EL ink was spin-coated on top of the patterned electrolyte layer to form a 100-nm-thick layer. To retain well-defined groove edges and thus obtain sharp emission features, the EL ink must not dissolve or damage the patterned electrolyte layer. Similarly, to improve the contrast between the light-emitting pattern and background, the electrolyte layer should form a smooth surface to reduce the risk of unwanted pinhole light emission. A careful optical interrogation revealed that the electrolyte pattern was left intact following the deposition of the light-emission ink. The patterned bilayer was dried on a hotplate at 343 K for >12 h, after which Al was thermally evaporated as the reflective top electrode. Then, the device was encapsulated with an epoxy-attached glass plate18 before being transferred out from the N2-filled glove box for characterization under ambient air.
The devices were driven and measured by a Keithley 2400 source-measure unit (or by a battery and multimeter assembly), with ITO biased as the anode. The current density was defined as the current divided by the total written electrolyte-free area, i.e., the total pattern-groove length multiplied by the pattern-groove width. Images of the patterned devices were captured with a digital single-lens reflex camera equipped with a macro-lens, whereas microscopic details of the emission lines were recorded with an optical microscope. The temporal evolution of the emission line width was investigated by analyzing time-lapse photographs using a MATLAB script.
Results and discussion
Figure 2a presents a photograph of a device featuring a luminescent line-art pattern with the shape of a fish, as driven by a low voltage of 4 V. The line-art pattern was formed ‘freely’ in a single direct-write step of the electrolyte layer during the device fabrication, as schematically shown in the upper right inset. Because the emission shape corresponds directly to the written pattern, it can be easily adjusted to any preference or style. Non-connected and isolated features, such as the eye of the fish in Figure 2a, are emissive despite the employment of non-patterned electrodes. In fact, all written features, regardless of size and position, are found to emit light in a well-resolved manner, provided that they are positioned in between the two charge-injecting electrodes. Both the line-art pattern and emission color can be controlled and adjusted based on preference. Figure 2b shows a photograph of the line-art emission from three devices all driven by a voltage of 4 V but based on different EL compounds. In this example, the electrolyte was patterned through a simple back-and-forth motion of the stylus; each device emits with a different color (here, red, yellow and blue) based on the energy gap of its constituent EL compound.
(a) Photograph of the patterned emission from an 8 × 8 mm2 line-art bilayer LEC device. The ‘fish’ pattern was created by ‘drawing’ in the electrolyte layer with a syringe needle, as schematically shown in the upper right inset. (b) Photograph of a showcase setup indicating the broad range of emission colors and patterns available with the line-art technology. The dashed lines indicate the substrate edges, where the electrode contact points are located. All devices were driven by a constant voltage of 4 V.
The patterning technology is found to be notably robust and practical, as exemplified by the fact that we have successfully tested four different EL compounds and three electrolytes in a variety of combinations, allowed the thickness of the EL layer to vary from 10 to 100 nm, and invariably utilized air-stabile materials for both the electrodes and bilayer. In fact, all investigated devices within the project have featured well-resolved and highly emissive line-art patterns at low drive voltage.
We now consider how these line-art bilayer LECs operate. Some input into the operational mechanism is provided in that the emission intensity and device current are observed to increase over time during an initial turn-on period (see Supplementary Fig. S1 for a graph on the temporal evolution of the current density). A conventional single-layer LEC device comprises a uniform and intimate blend of a conjugated organic EL compound and mobile ions as the active material19,20. When a voltage is applied to the electrodes, the ions begin to migrate to perform two critical tasks: First, the ions drift to the electrode interfaces to form electric double layers, which facilitate the efficient and balanced injection of holes and electrons into the EL compound, regardless of the energy barrier height at the electrode interfaces. Second, the electronic injection results in the formation of space charge, which is effectively neutralized by a further redistribution of the ions. The latter process is termed electrochemical doping, and n-type doping, i.e., cation compensation of injected electrons, takes place at the cathode, whereas p-type doping, being anion-compensation of injected holes, occurs at the anode. These highly conducting doped regions grow in size over time, and after a turn-on period, a p–n junction has formed in the bulk of the active material21,22,23. A distinct optoelectronic signature of this in situ doping process is an increase of current and luminance with time during device turn-on; hence, the line-art bilayer LECs also feature electrochemical doping.
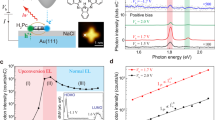
To investigate why the patterned features emit light instead of the entire device surface, as in conventional single-layer LECs, we have performed a microscopy study on the temporal and spatial evolution of the light-emission area in a region surrounding a written electrolyte-free groove. Representative optical micrographs of the same region before device turn-on (upper image), during turn-on (middle image), and at steady-state (lower image) are presented in Figure 3a. An important observation is that the entire 60-µm-wide stylus-written electrolyte-free groove does in fact not emit light; instead, the light emission is confined to two spatially separated regions located at the electrolyte edges. Moreover, the emission is initiated at the electrolyte edge, after which it primarily grows in the lateral direction of the non-patterned electrolyte, i.e., away from the written electrolyte-free groove. A revisit and closer inspection of the photographs displayed in Figure 2 reveals that these emission lines also consist of two distinct lines separated by a non-emissive central region, i.e., a ‘double line’, although the emission lines appear as a homogenous single line to the naked eye because we have opted to keep the groove width small.
(a) Three micrographs of the same region encompassing a 60-µm-wide written electrolyte-free line, as recorded at the following times after voltage application (from top to bottom): t = 0 s, t = 20 s, and t = 120 s. The micrographs were captured through the substrate side using episcopic illumination, and the device was driven by 6 V. (b) Time evolution of the emission line width from the same device, with the standard deviation indicated by the error bars. (c) Schematic of the device operation of the line-art bilayer LEC, with the open circles representing holes and the solid circles representing electrons. The solid lines with arrows indicate electronic and ionic motion during operation, whereas the dashed and crossed arrows denote unlikely transport paths. For clarity, the dimensions are not to scale.
A quantitative measure of the growth of the emission line width with time is presented in Figure 3b. The emission line width grows steadily during the first 2 min of operation, and for this particular device geometry, the average line width thereafter stabilizes at a steady-state value of 3 µm. However, the emission line width can probably be controlled by the material selection and/or bilayer architecture. Nevertheless, ultrahigh-resolution emission features can clearly be realized with the patterning process.
With this information at hand, we present a schematic of our view on the operational mechanism of the line-art bilayer LEC in Figure 3c. The fundamental criterion for light emission is that electrons (solid circles) and holes (open circles) are both injected and thereafter meet and recombine radiatively in the EL compound. We designed the device such that the anode was coated with an electronically blocking electrolyte layer, with the consequence that hole injection is prohibited from the electrolyte-coated regions (assuming that the electrolyte layer is compact and pinhole-free)24. By removing parts of the electrolyte layer with the stylus during the patterning process, we opened up a path for hole injection in the stylus-written, electrolyte-free grooves (as indicated by the solid arrows). According to the same argumentation, pinholes in the hole-blocking electrolyte layer could result in light emission also from the non-patterned regions, and a sprinkled weak background light was indeed also observed from some devices (see, e.g., Figure 2a).
The device was further designed to feature an air-stabile cathode, with the consequence that for most organic EL compounds, including those utilized in this study, a large barrier for electron injection exists at the cathodic interface. One way of decreasing the width of this barrier, and hence allowing for efficient electron injection through tunneling, is through the formation of an electric double layer at the cathode via the accumulation of cations. In this particular bilayer configuration, the double-layer formation requires that the cations be able to exit the electrolyte layer and move through the thickness of the EL compound layer24,25,26,27.
However, if this electric double-layer formation through cation accumulation took place over the entire cathodic interface, we would expect that the complete stylus-written and electrolyte-free region should light up because both electrons and holes then could be injected into this entire area. However, considering that light is found to be emitted only at the electrolyte edges (see Figure 3a), we draw the conclusion that the lateral motion of cations is hindered, and that a cathodic electric double layer only forms in, or extremely close to, the electrolyte-covered regions. The latter observation can be rationalized by the following: (i) the cations are driven by an electric field in the vertical inter-electrode direction but are only diffusing in the lateral direction, and (ii) the distance a cation must traverse in the vertical direction is short (∼100 nm) compared to the width of the stylus-written electrolyte-free region (60 µm in the device presented in Figure 3a). For simplicity, we have omitted the electrochemical doping process from the above discussion but suggest that a gradually growing p-type doping region situated close to the electrolyte layer (as indicated by the curved solid arrow in Figure 3c) can rationalize the observed temporal growth of the emission region because of its high conductivity28.
In this pioneering report on line-art emission from a patterned bilayer LEC, our focus has been on demonstrating the concept and establishing the operational procedure; however, we now briefly discuss future opportunities. Electrolyte-induced side reactions have been shown to be a common cause for degradation in single-layer LECs18,29, and we suggest that the physical separation of the EL compound from the electrolyte (or rather the ion-solvating medium) in the bilayer architecture could result in a much-desired improved stability for future optimized devices. This study has been aimed at the attainment of personalized emissive patterns, but the demonstrated micrometer-sized features indicate that large-area out-coupling patterns could be formed directly in the electrolyte layer. More specifically, as the out-coupling of light is a significant performance limiter in planar surface-emitting devices, a low-cost formation of a designed, large-area out-coupling pattern in the electrolyte layer using, for example, an imprint mold could represent a viable path to more efficient LEC panels30.
At this early stage of development, we are already able to repeatedly produce line-art devices that emit light that is sufficiently bright to be easily visible under indoor illumination at a low drive voltage. Accordingly, we propose that the established capacity of the LEC technology for high-throughput and low-cost solution-processing on flexible substrates15,16,17, in combination with the herein demonstrated opportunity for robust personalized emission patterns, could represent an ideal match for emerging applications, such as active packaging, personalized emissive gadgets, and high-performance illumination panels.
Conclusions
In summary, we have developed a mask-free and direct-write technology, capable of delivering personalized EL line art, from a thin and lightweight device driven at a few volts. The device structure comprises a solution-processed electrolyte/EL-compound bilayer that is sandwiched between two air-stabile electrodes, and the light-emission pattern is formed by subtractive patterning of the electrolyte layer. We show that the operational procedure involves cation-assisted electron injection at the cathode, which, combined with electrolyte-blocked hole injection at the anode, allows for the attainment of high-resolution patterns with micrometer-thin emissive lines. We propose that such a low-cost and flexible line-art device, featuring a designed emission pattern and driven by a low-voltage supply, could be of interest for many personalized and efficient applications.
References
Sandström A, Edman L . Towards high-throughput coating and printing of light-emitting electrochemical cells: a review and cost analysis of current and future methods. Energy Technol 2015; 3: 329–339 .
Arias AC, MacKenzie JD, McCulloch I, Rivnay J, Salleo A . Materials and applications for large area electronics: solution-based approaches. Chem Rev 2010; 110: 3–24 .
Carmon A, Vronsky E . Method and system for patterning an organic light emitting diode display by printing. Patent US20070069634 A1 ; 2007.
OLED-info . Upgraded image lighting technology from ITRI. Updated 12 May 2015. http://www.oled-info.com/itri-upgrades-their-image-lighting-technology (accessed 24 June 2015).
Matsuzawa T, Aoki Y, Takeuchi N, Murayama Y . A new long phosphorescent phosphor with high brightness, SrAl2O4: Eu2+, Dy3+. J Electrochem Soc 1996; 143: 2670–2673 .
Bessière A, Jacquart S, Priolkar K, Lecointre A, Viana B et al. ZnGa2O4: Cr3+: a new red long-lasting phosphor with high brightness. Opt Express 2011; 19: 10131–10137 .
Hölsä J, Jungner H, Lastusaari M, Niittykoski J . Persistent luminescence of Eu2+ doped alkaline earth aluminates, MAl2O4: Eu2+. J Alloy Compd 2001; 323–324: 326–330 .
Bube RH . Luminescence and trapping in zinc sulfide phosphors with and without copper activator. Phys Rev 1950; 80: 655–666 .
Tada K, Onoda M . Patterned emission from polymeric light-emitting device realized by photo-irradiation in air. Thin Solid Films 2000; 363: 195–197 .
Konica-Minolta . Flexible OLED lighting. Updated 2015. http://www.konicaminolta.com/oled/products/index.html (accessed 24 June 2015).
OLED-info . Custom designed structured artwork OLED panels from Philips. Updated 14 July 2013. http://www.oled-info.com/philips-now-offer-your-own-design-structured-artwork-oled-panels-%E2%82%AC120 (accessed 24 June 2015).
Mauthner G, Landfester K, Köck A, Brückl H, Kast M et al. Inkjet printed surface cell light-emitting devices from a water-based polymer dispersion. Org Electron 2008; 9: 164–170 .
Zheng H, Zheng YN, Liu NL, Ai N, Wang Q et al. All-solution processed polymer light-emitting diode displays. Nat Commun 2013; 4: 1971.
Shimoda T, Morii K, Seki S, Kiguchi H . Inkjet printing of light-emitting polymer displays. MRS Bull 2003; 28: 821–827 .
Hernandez-Sosa G, Tekoglu S, Stolz S, Eckstein R, Teusch C et al. The compromises of printing organic electronics: a case study of gravure-printed light-emitting electrochemical cells. Adv Mat 2014; 26: 3235–3240 .
Sandström A, Dam HF, Krebs FC, Edman L . Ambient fabrication of flexible and large-area organic light-emitting devices using slot-die coating. Nat Commun 2012; 3: 1002 .
Asadpoordarvish A, Sandström A, Larsen C, Bollström R, Toivakka M et al. Light-emitting paper. Adv Funct Mat 2015; 25: 3238–3245 .
Asadpoordarvish A, Sandström A, Tang S, Granström J, Edman L . Encapsulating light-emitting electrochemical cells for improved performance. Appl Phys Lett 2012; 100: 193508 .
Matyba P, Andersson MR, Edman L . On the desired properties of a conjugated polymer-electrolyte blend in a light-emitting electrochemical cell. Org Electron 2008; 9: 699–710 .
Shoji TD, Zhu ZH, Leger JM . Characterizing ion profiles in dynamic junction light-emitting electrochemical cells. ACS Appl Mater Interfaces 2013; 5: 11509–11514 .
Pei QB, Yu G, Zhang C, Yang Y, Heeger AJ . Polymer light-emitting electrochemical-cells. Science 1995; 269: 1086–1088 .
van Reenen S, Matyba P, Dzwilewski A, Janssen RAJ, Edman L et al. A unifying model for the operation of light-emitting electrochemical cells. J Am Chem Soc 2010; 132: 13776–13781 .
Lenes M, Garcia-Belmonte G, Tordera D, Pertegás A, Bisquert J et al. Operating modes of sandwiched light-emitting electrochemical cells. Adv Funct Mat 2011; 21: 1581–1586 .
Lindh EM, Sandström A, Edman L . Inkjet printed bilayer light-emitting electrochemical cells for display and lighting applications. Small 2014; 10: 4148–4153 .
Sandström A, Matyba P, Inganäs O, Edman L . Separating ion and electron transport: the bilayer light-emitting electrochemical cell. J Am Chem Soc 2010; 132: 6646–6647 .
Liu J, Engquist I, Berggren M . Double-gate light-emitting electrochemical transistor: confining the organic p-n junction. J Am Chem Soc 2013; 135: 12224–12227 .
Liu J, Engquist I, Crispin X, Berggren M . Spatial control of p-n junction in an organic light-emitting electrochemical transistor. J Am Chem Soc 2012; 134: 901–904 .
Hu YF, Gao J . Direct imaging and probing of the p-n junction in a planar polymer light-emitting electrochemical cell. J Am Chem Soc 2011; 133: 2227–2231 .
Fang JF, Matyba P, Robinson ND, Edman L . Identifying and alleviating electrochemical side-reactions in light-emitting electrochemical cells. J Am Chem Soc 2008; 130: 4562–4568 .
Saxena K, Jain VK, Mehta DS . A review on the light extraction techniques in organic electroluminescent devices. Opt Mater 2009; 32: 221–233 .
Acknowledgements
The authors acknowledge financial support from the Swedish Foundation for Strategic Research, the Swedish Research Council, the Swedish Energy Agency, the Kempe Foundation, the Knut and Alice Wallenberg Foundations, and Åforsk.
Note: Accepted article preview online 25 November 2015
Author information
Authors and Affiliations
Corresponding author
Additional information
Note: Supplementary Information for this article can be found on the Light: Science & Applications' website.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 Unported License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Lindh, E., Sandström, A., Andersson, M. et al. Luminescent line art by direct-write patterning. Light Sci Appl 5, e16050 (2016). https://doi.org/10.1038/lsa.2016.50
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/lsa.2016.50
Keywords
This article is cited by
-
Light-Emitting Electrochemical Cells: A Review on Recent Progress
Topics in Current Chemistry (2016)