Abstract
The expression of mucin (MUC2) in prostate cancer has not been well studied previously and may be of prognostic and pathobiologic significance. It is, however, well known that MUC2 expression in mucinous pancreatic and breast cancer represents an indolent pathway since these tumors have a significantly better outcome than their conventional counterparts. Twenty-five cases each of Gleason pattern 3 and 4 mucinous adenocarcinoma of the prostate defined by greater than 25% mucinous component and nonmucinous adenocarcinoma of the prostate were obtained from the surgical pathology files of the Johns Hopkins Hospital and Emory University Hospital. Immunohistochemical stains were performed for MUC2 on all 50 cases. Mean patient age was 60 years (range 44–72 years). MUC2 was expressed in all 25 cases (100%) of mucinous adenocarcinoma of the prostate, irrespective of the Gleason pattern. The nonmucinous component of these cases was negative for MUC2. In contrast, MUC2 expression was significantly lower in nonmucinous adenocarcinoma of the prostate, detected in only 6/25 cases as a focal finding, while 19/25 (76%) of nonmucinous adenocarcinoma of the prostate were completely negative for MUC2 (P<0.01). In six cases that showed focal positivity, MUC2 was expressed in areas with Gleason pattern 3 cancer with extensive mucinous fibroplasia (one case) and prominent intraluminal mucin (five cases). Other areas of these tumors were negative for MUC2. Mucinous adenocarcinoma of the prostate shows diffuse expression of MUC2, a known tumor suppressor, which is not present in either normal prostate or the majority of conventional adenocarcinomas of this organ. This indicates that mucinous adenocarcinoma of the prostate is indeed of the ‘colloid type’ akin to those in other exocrine organs. It is highly conceivable that this de novo expression of MUC2 has a role, not only in the mucinous differentiation of these tumors and their colloid pattern, but also in their relatively indolent behavior that has been recently elucidated.
Similar content being viewed by others
Main
Prostate cancer is the most common noncutaneous cancer in men worldwide.1, 2, 3 The vast majority of prostate cancers are acinar ‘nonmucinous’ adenocarcinomas.4 Mucinous adenocarcinoma of the prostate, also referred to as colloid adenocarcinoma, is rare and one of the least common morphologic variants of prostate cancer.4, 5, 6, 7, 8, 9 The incidence of mucinous adenocarcinoma of the prostate, defined by the presence of more than 25% extravasated mucin, is approximately 0.2%.6, 7, 9, 10 In exocrine organs, including breast, skin and pancreas, the expression of MUC2 (the goblet-type mucin) is limited to mucinous colloid-type carcinomas, not seen in conventional adenocarcinomas of these organs, or their usual type preinvasive precursors.11, 12, 13 In these organs, colloid carcinoma (pure mucinous carcinoma), characterized by well-circumscribed lakes of mucin that contain scanty, detached malignant cells has a significantly better prognosis than conventional ductal carcinomas.11 We sought to determine the expression of MUC2 in both mucinous adenocarcinoma of the prostate and nonmucinous adenocarcinoma of the prostate in lieu of the prognostic and pathobiologic significance of MUC2 expression in other previously described organs.
Materials and methods
Case Selection
Twenty-five cases each of radical prostatectomy specimens with Gleason patterns 3 and 4 mucinous adenocarcinoma of the prostate defined by greater than 25% mucinous component (of the entire tumor in the specimen) and nonmucinous adenocarcinoma of the prostate were obtained from the surgical pathology files of the Johns Hopkins Hospital and Emory University Hospital.
Immunohistochemistry
Immunohistochemical stains were performed for MUC2 on all 50 cases. Immunohistochemical stains were performed using the avidin–biotin–perixidase methodology. The primary immunohistochemical stain is a monoclonal antibody: MUC-2 (Glycoprotein clone: Ccp58 Novocastra, Newcastle upon Tyne, UK), used at 1/100 dilution. Antigen retrieval was performed at pH 6 in citrate buffer, in a pressure cooker for 3 min at a pressure of 15 pounds per square inch. The EnVision plus dual link detection kit (Dako Corp, Carpinteria, CA) was used. Positive stain was present in the cytoplasm. Normal large bowel was used as the positive control. Negative controls had specific antibody replaced by buffer.
Results
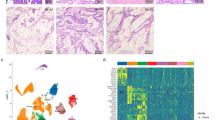
Mean patients age was 60 years (range 44–72 years). MUC2 was expressed in all 25 cases (100%) of mucinous adenocarcinoma of the prostate, irrespective of the Gleason pattern, characterized by diffuse intracytoplasmic staining (Figures 1a and 2b). The nonmucinous component of these cases was negative for MUC2 (Figure 3a and b). In contrast, MUC2 expression was of significantly lower frequency in nonmucinous adenocarcinoma of the prostate, detected in only 6/25 (24%) cases as a focal finding, while 19/25 (76%) cases were completely negative for MUC2 (P<0.01; Figure 4a and b). In six cases that showed focal positivity for MUC2, staining was in areas with Gleason pattern 3 cancer with extensive mucinous fibroplasia (one case) (Figure 5a and b) or with prominent intraluminal mucin (five cases) (Figure 6a and b). Other areas of these tumors were negative for MUC2. In all 50 cases (100%), MUC2 was negative in benign prostate glands.
(a) Mucinous adenocarcinoma of the prostate with cribriform pattern 4 with adjacent nonmucinous Gleason pattern 3 prostate cancer. (b) Positive MUC2 expression in cribriform pattern 4 mucinous prostate cancer and negative in adjacent Gleason pattern 3 nonmucinous prostate cancer, corresponds to focus in panel a.
Discussion
MUC2 was initially isolated from a human jejunum cDNA library and is thought to be one of the major intestinal mucin genes. MUC2 is a mucin epitope referred to as ‘gel forming’ or ‘secretory’ and its expression in normal tissues is largely limited to intestinal goblet cells, though it is also expressed in the trachea.14 One of its key functions is to form disulfide bonds and unusually large mucin globules. Although MUC2 is not typically expressed in the breast or pancreas, its expression has been previously documented in mucinous/colloid carcinomas of both organs, but not in their conventional ductal counterparts.11, 15, 16, 17
It has been shown that MUC2 expression is highly specific for mucinous/colloid carcinoma, especially in the breast and pancreas. In a study by Adsay et al,11 MUC2 labeling was detected in all 30 and 18 cases of mammary and pancreatic colloid carcinomas, respectively. In their study, conventional ductal carcinomas of these organs very rarely expressed this marker (3 of 44 (7%) and 1 of 136 (1%), respectively). Other papers have also demonstrated this specificity of MUC2 for colloid carcinoma.17, 18 Studies have also shown that the mucin in colloid carcinoma is distinctive, showing o-acylated forms of sialomucin, not generally formed in other carcinomas.11, 19
The specific association of MUC2 with colloid is likely to have a key role in the morphogenesis of this selected group of tumors. In normal tissue, the expression of this marker is largely limited to intestinal goblet cells, hence the name ‘secretory’-type mucin. MUC 2 is also known to have specific adhesive properties. It functions as a selective physiologic barrier because of its tendency to form large chains of glycoproteins by disulfide linkage, forming a gelatinous film lining the intestinal epithelium, hence its other synonym ‘gel-forming’ mucin. Therefore, it is no surprise that it may be a key factor in the morphogenesis of ‘gelatinous’ (colloid) carcinoma. In addition to accounting for the distinctive morphology of these neoplasms, MUC2 may also be a biological factor in the relatively slow growth of colloid carcinoma.11 The fact that MUC2 has been found to have tumor suppressor function is also suggestive of this hypothesis.20 Absence of MUC2 induces increased cell proliferation, decreased apoptosis and increased migration of intestinal epithelial cells, ultimately leading to a spectrum of neoplastic transformation, ranging from aberrant crypt foci to adenomas to frank carcinomas.11, 20 The findings of Adsay et al11 suggest that, along with its tumor suppressor activity documented in the gastrointestinal tract, the exocrine pancreas and the breast, MUC2 may have a role in diverting the process of carcinogenesis toward a more favorable prognostic pathway.
Mucinous adenocarcinoma of the prostate of the prostate, similar to usual acinar adenocarcinoma, is associated with elevated serum prostate-specific antigen (PSA) values, metastasize to bone and respond to hormonal therapy.21, 22 However, its prognosis relative to typical acinar carcinoma remains controversial with divergent data based on isolated case reports or small case series.5, 8, 23, 24, 25, 26
We recently showed in a study of 47 cases of mucinous adenocarcinoma of the prostate that this group of patients tends to do better, stage for stage and grade for grade, than patients with conventional nonmucinous adenocarcinoma of the prostate treated by radical prostatectomy.27 The mean follow-up of mucinous adenocarcinoma of the prostate patients without progression was 5.6 years (median 6 years, range: 1–15). One patient (2%) had PSA progression 3 years after his radical prostatectomy, without the evidence of local recurrence or distant metastasis. The 5-year actuarial PSA progression-free rate was 97% for all patients with mucinous adenocarcinoma of the prostate. Using the Kattan nomogram, the predicted mean 5-year actuarial PSA progression-free rate for nonmucinous adenocarcinoma of the prostate with the same PSA and postoperative findings as those with mucinous adenocarcinoma of the prostate in the study was 85%.27
This study has demonstrated, from the immunohistochemical standpoint, that MUC2 is expressed predominantly in mucinous adenocarcinoma of the prostate. Cappello et al28 studied cases of conventional prostate cancer with PCNA, p53, HSP60, HSP10 and MUC2. Their study showed that while PCNA, p53, HSP60 and HSP10 were variably expressed depending on the grade and stage of prostate cancer carcinogenesis, MUC2 was negative in both tumoral and nontumoral prostatic tissue.
Cozzi et al29 examined the expressions MUC1, MUC2, MUC4, MUC5AC and MUC6 in prostate cancer tissues using tissue microarrays (TMAs). There was overexpression of MUC1 in 58% of primary prostate cancer and 90% of lymph node metastases, but no expression in normal prostate or benign tissues. The expression of MUC2, MUC4, MUC5AC and MUC6 was found to be negative in both normal and neoplastic tissues. They, however, did not specify whether they had any cases of mucinous adenocarcinoma of the prostate.
In summary, to the best of our knowledge, our study is the largest to date, comparing the immunophenotypic profile of primary mucinous adenocarcinoma of the prostate and nonmucinous adenocarcinoma of the prostate in radical prostatectomy specimens. It is highly conceivable that this de novo expression of MUC2 has a role, not only in the mucinous differentiation of prostate cancers and their colloid pattern, but also in their relatively indolent behavior. Additional studies are warranted from the molecular biologic standpoint to further our understanding of this unique variant of prostate cancer.
References
Cooperberg MR, Moul JW, Carroll PR . The changing face of prostate cancer. J Clin Oncol 2005;23:8146–8151.
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66.
Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108.
Grignon DJ . Unusual subtypes of prostate cancer. Mod Pathol 2004;17:316–327.
Cricco RP, Kassis J . Mucinous adenocarcinoma of prostate. Urology 1979;14:276–278.
Dhom G . Unusual prostatic carcinomas. Pathol Res Pract 1990;186:28–36.
Epstein JI, Lieberman PH . Mucinous adenocarcinoma of the prostate gland. Am J Surg Pathol 1985;9:299–308.
Olivas TP, Brady TW . Mucinous adenocarcinoma of the prostate: a report of a case of long-term survival. Urology 1996;47:256–258.
Rhee AC, Olgac S, Ohori M, et al. Mucinous adenocarcinoma of the prostate: a case report of long-term disease-free survival and a review of the literature. Urology 2004;63:779–780.
Osunkoya AO, Epstein JI . Primary mucin-producing urothelial-type adenocarcinoma of prostate: report of 15 cases. Am J Surg Pathol 2007;31:1323–1329.
Adsay NV, Merati K, Nassar H, et al. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: Coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am J Surg Pathol 2003;27:571–578.
Hanski C, Hofmeier M, Schmitt-Graff A, et al. Overexpression or ectopic expression of MUC2 is the common property of mucinous carcinomas of the colon, pancreas, breast, and ovary. J Pathol 1997;182:385–391.
Siedow A, Szyf M, Gratchev A, et al. De novo expression of the Muc2 gene in pancreas carcinoma cells is triggered by promoter demethylation. Tumour Biol 2002;23:54–60.
Chambers JA, Hollingsworth MA, Trezise AE, et al. Developmental expression of mucin genes MUC1 and MUC2. J Cell Sci 1994;107:413–424.
Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol 2001;25:26–42.
Ho SB, Niehans GA, Lyftogt C, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res 1993;53:641–651.
O'Connell JT, Shao ZM, Drori E, et al. Altered mucin expression is a field change that accompanies mucinous (colloid) breast carcinoma histogenesis. Hum Pathol 1998;29:1517–1523.
Luttges J, Zamboni G, Longnecker D, et al. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol 2001;25:942–948.
Saez C, Japon MA, Poveda MA, et al. Mucinous (colloid) adenocarcinomas secrete distinct o-acylated forms of sialomucins: a histochemical study of gastric, colorectal and breast adenocarcinomas. Histopathology 2001;39:554–560.
Velcich A, Yang W, Heyer J, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002;295:1726–1729.
McNeal JE, Alroy J, Villers A, et al. Mucinous differentiation in prostatic adenocarcinoma. Hum Pathol 1991;22:979–988.
Teichman JM . Mucinous adenocarcinoma of the prostate: a report of a case of longterm survival. Urology 1996;48:660.
Lane BR, Magi-Galluzzi C, Reuther AM, et al. Mucinous adenocarcinoma of the prostate does not confer poor prognosis. Urology 2006;68:825–830.
Manne RK, Haddad FS . Mucinous adenocarcinoma of prostate. Urology 1989;33:247–249.
Randolph TL, Amin MB, Ro JY, et al. Histologic variants of adenocarcinoma and other carcinomas of prostate: pathologic criteria and clinical significance. Mod Pathol 1997;10:612–629.
Skodras G, Wang J, Kragel PJ . Primary prostatic signet-ring cell carcinoma. Urology 1993;42:338–342.
Osunkoya AO, Nielson ME, Epstein JI . Prognosis of Mucinous Adenocarcinoma of the Prostate Treated by Radical Prostatectomy. A Study of 47 Cases. Am J Surg Pathol 2008;32:468–472.
Cappello F, Rappa F, David S, et al. Immunohistochemical evaluation of PCNA, p53, HSP60, HSP10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Res 2003;23:1325–1331.
Cozzi PJ, Wang J, Delprado W, et al. MUC1, MUC2, MUC4, MUC5AC and MUC6 expression in the progression of prostate cancer. Clin Exp Metastasis 2005;22:565–573.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osunkoya, A., Adsay, N., Cohen, C. et al. MUC2 expression in primary mucinous and nonmucinous adenocarcinoma of the prostate: an analysis of 50 cases on radical prostatectomy. Mod Pathol 21, 789–794 (2008). https://doi.org/10.1038/modpathol.2008.47
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/modpathol.2008.47
Keywords
This article is cited by
-
Mucinous and secondary tumors of the prostate
Modern Pathology (2018)
-
Comparing nodal versus bony metastatic spread using tumour phylogenies
Scientific Reports (2016)