Abstract
Notwithstanding numerous evidences implicating toll-like receptor-4 (TLR4) in the pathogenesis of chronic hepatitis C virus (HCV) infection, the localization and level of TLR4 expression in the liver of patients with hepatitis C have never been investigated. We aimed to evaluate, by means of immunohistochemistry and real-time PCR (rt-PCR), hepatic TLR4 expression in patients with chronic HCV infection. Fifty patients who had undergone liver biopsy and 11 patients transplanted because of chronic HCV infection, and 12 controls free of liver disease, were included in the study. Each case was analyzed by immunohistochemistry for TLR4, α–smooth muscle actin and cytokeratin-7 (CK-7), and a subgroup of patients and all controls by rt-PCR for TLR4. Immunohistochemistry for α-smooth muscle actin was used to derive a score of activation of hepatic stellate cells and portal/septal myofibroblasts, while immunohistochemistry for CK-7 was used to evaluate and count hepatic progenitor cells, interlobular bile ducts and intermediate hepatocytes. In patients, the parenchymal elements responsible for the highest TLR4 level of expression were hepatic progenitor cells and biliary epithelial cells of interlobular bile ducts. Double-labeling experiments between anti-TLR4 and anti-CK7, anti-CD133, anti-CD44, anti-neural cell adhesion molecule, anti-epithelial cell adhesion molecule and anti-sex determining region Y-box 9, confirmed these findings. TLR4-positive hepatic progenitor cells and interlobular bile ducts were significantly correlated with the stage of liver disease (P<0.001), the grade of inflammation (P<0.001), and the activity of portal/septal myofibroblasts (P<0.001). rt-PCR study confirmed an increased TLR4 expression in the 26 patients analyzed with respect to controls (P<0.001). TLR4 expression positively correlated with fibrosis (P<0.05) and inflammation (P<0.05). The present results suggest that TLR4 expression by hepatic progenitor cells and biliary epithelial cells contributes to the progression of liver damage in the course of chronic HCV-related infection.
Similar content being viewed by others
Main
Innate and adaptive immune responses have a central role in the pathogenesis of hepatitis C virus (HCV) infection.1 The interplay between the virus and the host immune system determines the fate of the acute infection, ie, viral clearance or persistence, and the speed of damage progression in case of chronicity. Indeed, several evidences suggest that HCV is not directly cytopathic, and underscore the relevance of immune-mediated liver cell damage.2
Toll-like receptors (TLRs) comprise a highly conserved family of receptors that recognize pathogen-associated molecular patterns and allow the host to detect microbial infections. TLR4 is a transmembrane pattern-recognition receptor that has a key role in innate immunity by triggering inflammatory responses to its main ligand, Gram-negative bacteria lipopolysaccharide.3 Located between the portal and systemic bloodstream, the liver is exposed to extraordinarily high endotoxin concentrations and is the main clearance organ for lipopolysaccharide, which is taken up from portal blood by both Kupffer cells and hepatocytes, and excreted in large amounts in the bile.4, 5, 6 Therefore, it is not surprising that TLR4 has been implicated in the pathogenesis of most the liver diseases.7
Interaction between HCV and TLR4 signaling is strong although complex: HCV infection can directly induce TLR4 expression,8 and loss of tolerance to TLR ligands by monocytes/macrophages.9 Moreover, TLR4 signaling itself may regulate HCV replication.10 Notably, multiple variants in the TLR4 gene were found to modulate the risk of liver fibrosis in the Caucasian patients with chronic HCV infection.11, 12 More recently, in an experimental model, TLR4 was shown to mediate the synergism between HCV and alcohol in the liver damage, and to be implicated in liver oncogenesis in the hepatic progenitor cell transplantation model.13
However, only two previous studies have partially examined the localization and level of TLR4 protein expression in the liver of patients with HCV infection, one which generically aimed to verify the hepatic expression of all TLRs in the children with hepatitis C,14 and another in which primary biliary cirrhosis was under investigation and chronic HCV patients served as controls.15 Therefore, we aimed to evaluate the possible contribution of TLR4 in the pathogenesis of HCV-related liver damage by analyzing TLR4 expression in the liver of patients with chronic hepatitis C.
Patients and methods
Patients
Fifty patients who had undergone liver biopsy for chronic hepatitis C at the Campus Bio-Medico Hospital of Rome, and 11 patients who had been transplanted because of decompensated HCV-related liver cirrhosis, were included in the study. Inclusion criteria for all cases were: (1) patients had signed informed consent for the use of part of liver tissue for research studies before liver biopsy or surgery; (2) availability of complete clinical data and paraffin-embedded liver tissue from biopsy specimens or explanted liver. Exclusion criteria were: (1) history of alcohol abuse or coinfection with hepatitis B or human immunodeficiency virus; (2) diagnosis of hepatocellular carcinoma. Considering the above-mentioned literature, supporting a role for TLR4 in hepatic fibrogenesis,11, 12 the sample of patients who had undergone liver biopsy was selected in order to obtain a homogeneous representation of all stages of fibrosis: 19 patients with mild (stages 0, 1 and 2), 18 patients with moderate (stages 3 and 4), and 13 patients with severe fibrosis, ie, histological cirrhosis (stages 5 and 6), according to the Ishak's staging system.16
Twelve patients with normal transaminases, testing negative for HBsAg and anti-HCV antibodies, and presenting histologically normal liver were included in the study as a control group. They were six cadaveric liver donors and six patients who had undergone liver surgery for metastatic tumors (two colon cancers, two biliary cystadenomas, one gastric cancer, one endocrine tumor). In metastatic patients, liver tissue for immunohistochemistry and real-time PCR (rt-PCR) was at least 0.5 cm from the metastatic lesion.
Paraffin-embedded liver tissue for histology and immunohistochemistry was available from all the patients and controls, and fresh-frozen liver tissue for rt-PCR analyses was available from the explanted livers of the 11 transplanted patients, from liver biopsy samples of 15 patients, and from all controls.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local Ethics Committee.
Clinical Data and Liver Pathology
Epidemiological and biochemical data were collected from clinical records at the time of liver biopsy or liver transplantation. Hematoxylin/eosin and Masson's thricrome or Sirius red staining from each case were reassessed by a single operator (S.C.), and scored according to the Ishak's classification.16
Immunohistochemistry and Immunofluorescence
Besides TLR4, each case was analyzed by immunohistochemistry for α-smooth muscle actin and cytokeratin-7 (CK7). Immunohistochemistry was performed on 3–5 μm thick sections obtained from formalin-fixed tissue embedded in paraffin. Antigen retrieval was performed using a PT module (pH 6; Thermo Fisher Scientific, Fremont, CA, USA). For the identification of interlobular bile ducts, bile ductules and hepatic progenitor cells, the primary antibody used was anti-CK7 (dilution 1/200; mouse monoclonal, clone OV-TL 12/30, DakoCytomation, Botany, New South Wales, Australia), and for the identification of activated hepatic stellate cells and portal/septal myofibroblasts the primary antibody used was anti-α-smooth muscle actin (dilution 1/400; mouse monoclonal, clone 1A4, DakoCytomation). The immunohistochemical procedure was performed in a Thermo Autostainer 360-2D System (Histocom, Wiener Neudorf, Austria), using the UltraVision LP detection system (Thermo Fisher Scientific) and DAB Plus (Thermo Fisher Scientific) as a chromogen. Immunohistochemical staining for TLR4 was performed with the streptavidin–biotin method using LSAB system anti-mouse/anti-rabbit/anti-goat (Dako, Glostrup, Denmark). The primary antibody used was anti-TLR4 overnight incubated (dilution 1/50; goat polyclonal, Abcam, Cambridge, UK). Light microscopy micrographs were captured by a Videocam (SPOT Insight; Diagnostic Instrument, Sterling Heights, MI, USA) connected to an Olympus BX-51 light microscope (Olympus, Tokyo, Japan) and processed with an Image Analysis System (Delta Sistemi, Rome, Italy).
Immunofluorescent staining was performed on sections from paraffin-embedded tissue with the same anti-TLR4 and anti-CK7 antibodies used for immunohistochemistry, and with the anti-CD133 antibody (dilution 1/100; mouse monoclonal, clone AC133, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), the anti-CD44 antibody (dilution 1/40; mouse monoclonal, clone DF1485, Novocastra, Leica System, Newcastle Upon Tyne, UK), the anti-neural cell adhesion molecule (NCAM, also known as CD56) antibody (dilution 1/25; mouse monoclonal, clone 123C3, Santa Cruz Biotechnology, Santa Cruz, CA, USA), the anti-epithelial cell adhesion molecule (EpCAM) antibody (dilution 1/400; mouse monoclonal, clone C-10, Santa Cruz Biotechnology), and the anti-sex determining region Y-box 9 (SOX9) antibody (dilution 1/500; mouse monoclonal, Abcam), as other markers for hepatic progenitor cells.17, 18 In order to perform double-labeling experiments, species-specific DyLight 488/Cy5-conjugated secondary antibodies (dilution 1/200; Jackson ImmunoResearch Europe, Suffolk, UK) or Alexa Fluor 488/568-conjugated secondary antibodies (dilution 1/100; Invitrogen, Carlsbad, CA, USA) were used. Nuclear counterstaining was performed using Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) containing 4,6-diamidino-2-phenylindole or propidium iodide (Molecular Probes, Eugene, OR, USA). Fluorescence images were collected with a Nikon A1 Confocal Laser Microscope System (Nikon, Tokyo, Japan). Acquisition was carried out using the Imaging Software NIS-Elements (Nikon).
Negative control slides processed with normal serum were included for each staining. All measurements were performed simultaneously by two observers without knowledge of the patient's data, using a double-headed microscope.
Histological Scores
Perisinusoidally located stellate-shaped cells residing in the parenchymal lobules or nodules were named hepatic stellate cells.19 Stellate- or spindle-shaped cells at the interface between parenchyma and portal tract or between parenchyma and septa, and those residing in the portal tracts and the fibrotic septa, were named portal/septal myofibroblasts.19 Immunohistochemistry for α-smooth muscle actin was used to derive a score of activation of portal/septal myofibroblasts and hepatic stellate cells (Figures 1a–d), as already described by Schmitt-Graff et al.20 Immunoreactivity was scored arbitrarily as follows: 0= no positivity; 0.5= positivity of <10% of portal/septal myofibroblasts; 1= positivity of 10–20% of portal/septal myofibroblasts; 2= positivity of 20–50% of portal/septal myofibroblasts; and 3= positivity of >50% of portal/septal myofibroblasts. Concerning hepatic stellate cells: 0= no staining; 0.5= staining of some hepatic stellate cells occupying approximately <1% of the sinusoidal liver cell surface; 1= staining of hepatic stellate cells occupying ∼1–10% of the sinusoidal liver cell surface; 2= staining of hepatic stellate cells occupying ∼10–30% of the sinusoidal liver cell surface; and 3= staining of hepatic stellate cells occupying >30% of the sinusoidal liver cell surface. Hepatic progenitor cells, interlobular bile ducts, and intermediate hepatocytes were evaluated by immunohistochemistry for CK7 (Figures 2a–d). The hepatic progenitor cell compartment was quantified by counting small, CK7-positive cells in the periportal area of the lobule and by expressing them as the number per field.21 The cells were present as single cells, strings representing canals of Hering, and bile ductules of ductular reaction. Counted cells were smaller than normal hepatocytes and showed cytoplasmic staining.22 The interlobular bile ducts were separately counted as forming lumen elements constituted by CK7-positive cells. Morphologically typical hepatocytes with anti-CK7-positive cytoplasmic staining were considered intermediate hepatocytes,22 and were also separately evaluated. For each case, mean values of hepatic progenitor cells, interlobular bile ducts, and intermediate hepatocytes were obtained by counting the three fields at × 10 magnification (one field: 922.04 × 688.20 μm) with the highest anti-CK7 intensity of staining.
Immunohistochemistry for α-smooth muscle actin in hepatitis C virus (HCV)-related chronic hepatitis. Immunohistochemistry for α-smooth muscle actin was used to derive a score of activation of portal/septal myofibroblasts and hepatic stellate cells in mild (a), moderate (b), severe (c) fibrosis, and decompensated cirrhosis (d). Proceeding from mild fibrosis to decompensated cirrhosis, an increasing activation of portal/septal myofibroblasts and hepatic stellate cells was observed. Original magnification, × 100. Calibration bar: 50 μm.
Immunohistochemistry for cytokeratin-7 (CK7) in hepatitis C virus (HCV)-related chronic hepatitis. Hepatic progenitor cells, interlobular bile ducts and ductules, and intermediate hepatocytes were evaluated by immunohistochemistry for CK7 in mild (a), moderate (b), severe (c) fibrosis, and decompensated cirrhosis (d). Proceeding from mild fibrosis to decompensated cirrhosis, increasing numbers of hepatic progenitor cells, interlobular bile ducts and ductules, and intermediate hepatocytes were observed. Original magnification, × 100. Calibration bar: 50 μm.
After having verified that the parenchymal elements with the highest anti-TLR4 intensity of staining were hepatic progenitor cells and cells of the biliary lineage, mean values of anti-TLR4-positive hepatic progenitor cells and interlobular bile ducts were obtained by counting in the three fields at × 10 magnification (one field: 922.04 × 688.20 μm) with the highest anti-TLR4 intensity of staining and evaluating serial sections stained for CK7 in order to clarify whether TLR4-positive elements were hepatic progenitor cells, biliary epithelial cells, or hepatocytes. Concerning hepatic progenitor cells and biliary epithelial cells, they were considered positive when showing cytoplasmic and/or plasma membrane positivity, irrespective of the intensity of staining. Concerning hepatocytes, whose anti-TLR4 positivity—when present—was more homogeneously distributed, the mean intensity of staining was recorded and graded according to the following semiquatitative score: 0= no positivity; 1= mild intensity of staining; 2= moderate intensity of staining; and 3= high intensity of staining.
RNA Isolation, Reverse Transcriptase PCR and rtPCR
Total RNA was extracted using TRI Reagent (Sigma-Aldrich, St. Louis, MO, USA). RNA (2 μg) was treated with DNaseI (Gibco-BRL, Paisley, UK) before reverse transcription with M-MLV Reverse Transcriptase (Gibco-BRL) in the presence of RNaseOUT (Gibco-BRL). All primers were designed in order to distinguish between genomic and cDNA amplification, and all PCR products were sequenced to confirm the specificity. rt-PCR was performed with 1/20 of the reverse transcription reaction using an iCycler (BioRad, Hercules, CA, USA) and the iQ SYBR Green Supermix (BioRad) essentially as previously described.23 The primers used for TLR4 amplification were: sense 5′-CTGGGAGCCCTGCGTGGAGG-3′ and antisense 5′-AATTGTCTGGATTTCACACCTGG-3′. TLR4 mRNA levels were normalized according to histone H3 mRNA quantitation in the same sample. The primers used for histone H3 amplification were: sense 5′-AAAGCCGCTCGCAAGAGTGCG-3′ and antisense 5′-ACTTGCCTCCTGCAAAGCAC-3′. To monitor the specificity, final PCR products were analyzed by melting curves and electrophoresis. The amount of TLR4 transcript was calculated and expressed as the difference relative to the control gene histone H3 (2ΔCt, where ΔCt represents the difference in threshold cycles between the target and control genes).
Statistics
Depending on the parametric or nonparametric distribution, variables are expressed as mean±s.d. or median and 95% confidence interval (95% CI), respectively. In case of variables with a normal distribution, differences between subgroups were analyzed by one-way ANOVA eventually followed by the t-test for post-hoc analyses. For nonparametric variables, differences were evaluated by the Kruskal–Wallis test eventually followed by the Mann-Whitney U-test for post-hoc analyses. Categorical variables were compared by the χ2 test. Correlations were carried out by the Spearman's rank correlation test. A P<0.05 was considered statistically significant. SPSS software (version 17.00; SPSS, Chicago, IL, USA) was used for statistical analyses.
Results
As already indicated in the ‘Patients and methods’ section, 19 patients had a mild, 18 a moderate, and 13 a severe liver fibrosis, ie, histological liver cirrhosis, (stages 0–1–2, 3–4, and 5–6, respectively, according to the Ishak's classification), whereas 11 patients had undergone liver transplantation for decompensated liver cirrhosis. The 13 patients who received the diagnosis of cirrhosis after liver biopsy performed with the purpose of staging chronic liver disease had a fully preserved liver function and showed no complications from portal hypertension. Age (61.2±8.3 years) and sex (7M/5F) of the controls did not differ from those of patients (P=0.5 and P=0.9, respectively). Table 1 shows epidemiological, anthropometric, and biochemical data of the study population subanalyzed according to the stage of liver disease. No significant differences were observed among the different subgroups in terms of age, sex, and the anthropometric measures tested. The lack of age difference between groups reflects mainly the fact that only the younger subpopulation of cirrhotics is usually considered for the liver biopsy or can be included in a transplant list. There was a trend, although not significant, towards higher levels of HCV-RNA and higher prevalence of type 1 genotype in the more advanced stages of disease. As expectable, liver enzymes increased with advancing disease because of the strict correlation between necroinflammatory activity and fibrosis (see Table 2), while liver function tests were altered in cirrhotic patients.
Ishak's grade of inflammation, activity scores of hepatic stellate cells and portal/septal myofibroblasts, and mean counts of hepatic progenitor cells, interlobular bile ducts and intermediate hepatocytes in controls and in patients according to the stage of liver disease are reported in Table 2. The inflammatory score, the activity of portal/septal myofibroblasts, and the numbers of hepatic progenitor cells, interlobular bile ducts and intermediate hepatocytes showed a progressive increase with advancing stages of fibrosis, while the activity of hepatic stellate cells was not related with the stage of liver disease. As shown in Table 3, strong correlations were observed between the grade of inflammation, activity score of portal/septal myofibroblasts, number of hepatic progenitor cells, interlobular bile ducts, and intermediate hepatocytes.
In controls, a mild anti-TLR4 immunostaining was detected in hepatocytes and interlobular bile ducts, while TLR4-positive hepatic progenitor cells were not observed. In patients, the parenchymal elements responsible for the highest TLR4 staining intensity were hepatic progenitor cells/ductular reaction and biliary epithelial cells of interlobular bile ducts (Figures 3a–d). Double-labeling experiments between anti-TLR4 and anti-CK7, anti-CD133 (Figures 3e–f), anti-CD44, anti-NCAM, anti-EpCAM, anti-SOX9 (Figure 4) confirmed these findings. Anti-TLR4 immunostaining was also frequently observed in hepatocytes, and more consistent in periportal and/or periseptal hepatocytes in cases with advanced fibrosis (Figure 3d). As expected, many TLR4-positive elements were visualized among the cells constituting the inflammatory infiltrates, while we did not observe TLR4-positive cells with the morphology of hepatic stellate cells.
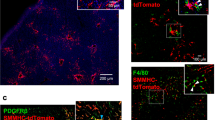
Immunohistochemistry for toll-like receptor-4 (TLR4) in hepatitis C virus (HCV)-related chronic hepatitis, and double-labeling experiments of TLR4 and cytokeratin-7 (CK-7), and of TLR4 and CD133. TLR4 positivity involves faintly scattered biliary epithelial cells (arrowheads) in cases with mild fibrosis (a). Increased number of TLR4-positive hepatic progenitor cells and biliary epithelial cells (arrowheads) were observed in cases with moderate (b) and severe (c) fibrosis, and the highest level of TLR4 expression was observed in biliary epithelial cells and hepatic progenitor cells of cases with decompensated cirrhosis (d), where some strongly TLR4-positive hepatocytes (arrow) were also found. Original magnification, × 100; calibration bar: 50 μm; high power field, × 400, (a–d). High-power micrographs of cirrhotic liver specimens labeled by double immunofluorescence. Immunofluorescent co-staining of TLR4 (green), and CK7 (red) as a marker of hepatic progenitor cells and biliary epithelial cells (e). Biliary epithelial cells (arrows) in interlobular ducts and hepatic progenitor cells (arrowheads) show TLR4 positivity, as well as neighborhood hepatocytes. In the mature biliary structures forming a lumen, TLR4 positivity involves both the cytoplasm and the apical membrane. Immunofluorescent co-staining of TLR4 (green), and CD133 (red) as a marker of hepatic progenitor cells (f). TLR4-positive ductules show anti-CD133 apical positivity denoting an immature phenotype. TLR4 was stained in green using an ALEXA 488-labeled secondary antibody, whereas CK7 and CD133 were stained in red using an ALEXA 568-labeled secondary antibody. Nuclear counterstaining was obtained with 4,6-diamidino-2-phenylindole. Original magnification, × 600; calibration bar: 20 μm, (e, f).
Double-labeling experiments of toll-like receptor-4 (TLR4) and hepatic progenitor cells specific markers. Representative images of TLR4-positive hepatic progenitor cells within ductular reaction. TLR4-positive elements show different degrees of cytoplasmic staining for CD44 (a-c), neural cell adhesion molecule (NCAM) (d-f), and epithelial cell adhesion molecule (EpCAM) (g-i), and nuclear staining for sex determining region Y-box 9 (SOX9) (j-l). Some hepatic progenitor cells can be detected also in the context of small lumen-forming bile ducts. TLR4 was stained in green using a DyLight 488-conjugated secondary antibody (a, d, g and j), whereas all other markers were stained in blue using a Cy5-conjugated secondary antibody (b, e, h and k). Merged images confirmed colocalization (arrowheads) (c, f, i and l). In panel c, a CD44-positive inflammatory cell is evidenced (arrow). Nuclear counterstaining was obtained with propidium iodide. Nuclear counterstaining images were not included in (j–l) in order to highlight nuclear SOX9 positivity. Original magnification, × 600; calibration bar: 10 μm.
In patients, there was a strong correlation between the stage of liver disease and TLR4-positive hepatic progenitor cells (ρ=0.82, P<0.001), and TLR4-positive interlobular bile ducts (ρ=0.68, P<0.001; Figure 5). For each case, the percentage of TLR4 positivity was obtained by dividing the mean numbers of TLR4-positive hepatic progenitor cells or TLR4-positive interlobular bile ducts by the total numbers of hepatic progenitor cells and interlobular bile ducts, respectively. Although the percentage of TLR4-positive bile ducts was no longer correlated with the stage of liver disease (ρ=0.18, P=0.2), a less strong but still highly significant correlation was found between the percentage of TLR4-positive hepatic progenitor cells and the stage of liver disease (ρ=0.49, P<0.001; Figure 5).
Toll-like receptor-4 (TLR4) expression and fibrosis. TLR4-positive hepatic progenitor cells and interlobular bile ducts, as absolute counts (a, b) or as percentages in relation to the total number of hepatic progenitor cells or interlobular bile ducts (c, d) according to the stage of chronic liver disease.
TLR4-positive hepatic progenitor cells and interlobular bile ducts were significantly correlated also with Ishak's grade of inflammation (ρ=0.74, P<0.001; ρ=0.60, P<0.001, respectively), mainly with the interface activity score of Ishak's grading (ρ=0.79, P<0.001; ρ=0.67, P<0.001, respectively), and with the activity of portal/septal myofibroblasts (ρ=0.63, P<0.001; ρ=0.67, P<0.001, respectively), but not with the activity of hepatic stellate cells (ρ=0.17, P=0.2; ρ=0.25, P=0.06, respectively; Figure 6). However, when the percentages were used in correlations, again, that of TLR4-positive hepatic progenitor cells, but not that of TLR4-positive interlobular bile ducts, were significantly correlated with the inflammatory score (ρ=0.42, P=0.001; ρ=0.1, P=0.48, respectively), with the interface activity score (ρ=0.41, P=0.001; ρ=0.25, P=0.07, respectively), with the activity of portal/septal myofibroblasts (ρ=0.48, P<0.001; ρ=0.24, P=0.07, respectively), and also weakly with the activity of hepatic stellate cells (ρ=0.32, P=0.02; ρ=0.20, P=0.14, respectively; Figure 6).
Toll-like receptor-4 (TLR4) expression, inflammation and activity of portal/septal myofibroblasts. TLR4-positive hepatic progenitor cells, as absolute counts (a-c), or as percentages in relation to the total number of hepatic progenitor cells (d-f), according to the grade of total inflammation, interface inflammation, and activity of portal/septal myofibroblasts. According to the 33th and 66th percentiles of data distribution: total inflammation was graded as mild (0–7), moderate (8–9), or severe (≥10) points of the Ishak's activity score; interface inflammation was graded as mild (0–1), moderate (2), or severe (3–4) points of the relative subclassification of the Ishak's score; activity of portal/septal myofibroblasts was graded as mild (0.5 or 1), moderate (2), or severe (3) points of the score described in the ‘Patients and methods’ section.
Finally, the score of hepatocyte TLR4 positivity was weakly correlated with the stage of chronic liver disease (ρ=0.31, P=0.02), and with the activity of portal/septal myofibroblasts (ρ=0.30, P=0.02), but not with the grade of inflammation (ρ=0.24, P=0.07), and with the activity of hepatic stellate cells (ρ=0.22, P=0.10).
The evaluation of TLR4 mRNA levels confirmed an increased TLR4 expression in the 26 patients analyzed with respect to controls (Figure 7). In patients, TLR4 expression was correlated with fibrosis (ρ=0.42, P<0.05) and Ishak's grade of inflammation (ρ=0.40, P<0.05). Notably, TLR4 expression by rt-PCR was not correlated with TLR4-positive hepatic progenitor cells (P=0.7), interlobular bile ducts (P=0.3), or hepatocytes (P=0.7), suggesting that inflammatory cells are the main contributors to the total amount of liver TLR4 mRNA. Therefore, differential TLR4 expression by parenchymal elements cannot be accurately examined by measuring the levels of TLR4 mRNA or protein in whole liver tissue extracts.
Toll-like receptor-4 (TLR4) expression by real-time PCR (rt-PCR). TLR4 mRNA levels in patients and controls (a), and according to the grade of total inflammation (b) and fibrosis (c). According to the median values in the 26 patients analyzed for rt-PCR, total inflammation was divided in mild/moderate (0–8) or severe (≥9) points of the Ishak's activity score; fibrosis was divided in pre-cirrhosis (stages 0–4) or cirrhosis (stages 5–6) according to the Ishak's staging system.
Discussion
Liver cell-type specific expression of TLR4 was herein evaluated in hepatic tissues obtained from patients with chronic HCV infection. TLR4 was found to be consistently expressed by hepatic progenitor cells/ductular reaction and by biliary epithelial cells of interlobular bile ducts, and TLR4 expression was significantly related to the grade of inflammation, mainly in its interface component, to the activation of portal/septal myofibroblasts, and to liver fibrosis. Therefore, the present results clearly suggest a role of TLR4 in the pathogenesis of HCV-related liver damage.
TLR4 is a transmembrane receptor recognizing lipopolysaccharide as its main ligand.3 Activation of TLR4 causes inflammation by promoting the secretion of inflammatory cytokines, such as tumor necrosis factor-α and interleukin-6, through the MyD88-dependent pathway, and anti-viral effects by promoting the secretion of interferon-β through the MyD88-independent pathway.24 Although the function of TLR4 in lipopolysaccharide-stimulated proinflammatory responses of Kupffer cells has been well characterized,8, 9 more recent findings suggest a direct role of TLR4 in hepatic fibrogenesis.25 In fact, TLR4 expression in hepatic stellate cells was found to enhance transforming growth factor-β signaling and hepatic fibrosis,26 and two TLR4 polymorphisms were associated with lowering of hepatic stellate cells apoptotic threshold and protection from fibrosis development.27
In the present study, we observed that in the advanced stages of liver disease, TLR4 was most prominently expressed by biliary epithelial cells of interlobular bile ducts and by the cells that we characterized as hepatic progenitor cells. Indeed, double-labeling experiments demonstrated the colocalization between TLR4 and CD133, CD44, NCAM, EpCAM, and SOX9, which were recently proposed as hepatic progenitor cell markers,17, 18 clearly demonstrating that hepatic progenitor cells contribute to a significant amount of hepatic TLR4 expression.
When interpreting TLR4 expression by biliary epithelial cells and hepatic progenitor cells, it is fundamental to consider lipopolysaccharide metabolism, wherein the liver has a central role. Indeed, because of its collocation between the portal and systemic circulations, the liver is exposed to very high lipopolysaccharide levels, representing the main contributor to lipopolysaccharide clearance. Kupffer cells were initially claimed as the only responsible for lipopolysaccharide uptake from the portal blood, but it was subsequently demonstrated that significant amounts of endotoxin are taken up by hepatocytes and secreted into the biliary system.5, 6 Therefore, both hepatic progenitor cells, which reside in the canals of Hering at the connection between the bile canalicular system and the interlobular bile ducts,28 and biliary epithelial cells are exposed to significant lipopolysaccharide concentrations. Endotoxin levels are further increased in the course of chronic liver disease due to changes in intestinal mucosal permeability and increase in bacterial translocation.4 In fact, a significant accumulation of lipopolysaccharide has been demonstrated in the biliary epithelial cells from the patients with HCV-related chronic hepatitis and liver cirrhosis by immunohistochemistry for lipid A, a common structural determinant and the biologically active part of lipopolysaccharide.29
We found a significant correlation between TLR4 expression by hepatic progenitor cells and biliary epithelial cells, and grade of inflammation, mainly interface activity, activation of portal/septal myofibroblasts, and liver fibrosis. Hepatic progenitor cells are bipotential stem cells residing in the human and animal livers that are able to differentiate towards the hepatocytic and the cholangiocytic lineages, whose proliferation leads to the so called ductular reaction.22, 28 Consistent with the typical finding in biliary diseases, where ductular reaction is considered the pacemaker of liver fibrosis,30 the activation of the hepatic progenitor cell compartment and the consequent ductular reaction have been shown to be associated also with the severity of nonbiliary chronic liver disease.31 Studies in patients with biliary disorders and in experimental models of biliary fibrosis have shown that the ductal epithelium can express several profibrogenic and chemotactic proteins, the latter capable of attracting and activating cells of both inflammatory and stellate cell lineages.32, 33, 34 In this context, the proinflammatory cytokines produced in response to TLR4 signaling could participate in the cross-talk between hepatic progenitor cells and proliferating cholangiocytes on the one side, and inflammatory cells and portal/septal myofibroblasts on the other side. Moreover, lipopolysaccharide has been shown to directly induce cholangiocyte proliferation,35 and, more recently, to induce the proliferation of hematopoietic progenitors,36 bone marrow mesenchymal stem cells,37 embryonic stem cells, and adult tissue-specific stem cells/progenitors, ie, intestinal and mammary gland stem cells/progenitors.38 Given this background, our finding of an increased rate of TLR4 expression by hepatic progenitor cells with increasing liver damage suggests a direct role of TLR4 in the activation of the hepatic progenitor cell compartment. Consistent with this hypothesis, a very recent experimental study found that transplantation of p53-deficient hepatic progenitor cells transduced with TLR4 results in liver-tumor development in the mice following repetitive lipopolysaccharide injection.13 However, further studies are required in order to verify the role of TLR4 in hepatic progenitor cell activation, proliferation, and transformation.
rt-PCR analysis of TLR4 mRNA levels confirmed a significant TLR4 hyperexpression in the patients with chronic hepatitis C and the association between TLR4 expression, the grade of inflammation and the degree of fibrosis, supporting the role of TLR4 in the pathogenesis of HCV-related chronic liver disease. Notably, we did not observe a correlation between TLR4 expression by immunohistochemistry and rt-PCR. This is likely due to the fact that inflammatory cells are the main contributors to the whole-liver amounts of TLR4 mRNA and, in this context, differences in TLR4 expression by parenchymal elements can hardly be appreciated. Therefore, as demonstrated in the present study, rt-PCR and immunohistochemical analysis of TLR4 provide important and complementary informations. Although rt-PCR shows that TLR4 expression is associated with liver-damage progression in the patients with chronic hepatitis C, immunohistochemistry highlights, for the first time, one of the mechanisms that could determine this association. Further mechanisms, implicating TLR4 expression by other cells in the liver, are likely to be involved.
It is important to emphasize that we did not observe TLR4-positive hepatic stellate cells in our immunohistochemistry study. However, experimental studies in vitro and in vivo have already demonstrated that hepatic stellate cells do express TLR4, that lipopolysaccharide treatment of hepatic stellate cells increases their fibrogenic potential,26 and that TLR4 polymorphisms affect the apoptotic threshold of hepatic stellate cells.27 Clearly, our inability to detect TLR4 in hepatic stellate cells can be attributed to the sensitivity of the immunohistochemistry technique, and does not exclude the expression of this receptor in fibrogenic cells. At the most, it could suggest that the mechanisms emerging from the experimental studies need to be verified in the human clinical setting.
In conclusion, the present study is the first to demonstrate a significant TLR4 expression by hepatic progenitor cells and biliary epithelial cells of interlobular bile ducts in the patients with chronic hepatitis C, and the correlation between this expression and liver damage in terms of inflammation, activation of portal/septal myofibroblasts and fibrosis. These results, which need to be validated by further experimental studies, suggest an important pathophysiological mechanism underlying the well-known connection between the liver infection, inflammation, regeneration/repair and, possibly, cancer.
References
Freeman AJ, Marinos G, French RA, et al. Immunopathogenesis of hepatitis C virus infection. Immunol Cell Biol 2001;79:515–536.
Cerny A, Chisari FV . Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology 1999;30:595–601.
Beutler B . Inferences, questions and possibilities in toll-like receptor signalling. Nature 2004;430:257–263.
Schwabe RF, Seki E, Brenner DA . Toll-like receptor signaling in the liver. Gastroenterology 2006;130:1886–1900.
Van Bossuyt H, De Zanger RB, Wisse E . Cellular and subcellular distribution of injected lipopolysaccharide in rat liver and its inactivation by bile salts. J Hepatol 1988;7:325–337.
Mimura Y, Sakisaka S, Harada M, et al. Role of hepatocytes in direct clearance of lipopolysaccharide in rats. Gastroenterology 1995;109:1969–1976.
Seki E, Brenner DA . Toll-like receptors and adaptor molecules in liver disease: update. Hepatology 2008;48:322–335.
Machida K, Cheng KT, Sung VM, et al. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol 2006;80:866–874.
Dolganiuc A, Norkina O, Kodys K, et al. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology 2007;133:1627–1636.
Broering R, Wu J, Meng Z, et al. Toll-like receptor-stimulated non-parenchymal liver cells can regulate hepatitis C virus replication. J Hepatol 2008;48:914–922.
Huang H, Shiffman ML, Friedman S, et al. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology 2007;46:297–306.
Li Y, Chang M, Abar O, et al. Multiple variants in toll-like receptor 4 gene modulate risk of liver fibrosis in Caucasians with chronic hepatitis C infection. J Hepatol 2009;51:750–757.
Machida K, Tsukamoto H, Mkrtchyan H, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci USA 2009;106:1548–1553.
Mozer-Lisewska I, Sluzewski W, Kaczmarek M, et al. Tissue localization of toll-like receptors in biopsy specimens of liver from children infected with hepatitis C virus. Scand J Immunol 2005;62:407–412.
Wang AP, Migita K, Ito M, et al. Hepatic expression of toll-like receptor 4 in primary biliary cirrhosis. J Autoimmun 2005;25:85–91.
Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696–699.
Spee B, Carpino G, Schotanus BA, et al. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut 2010;59:247–257.
Turner R, Lozoya O, Wang Y, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011;53:1035–1045.
Cassiman D, Libbrecht L, Desmet V, et al. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol 2002;36:200–209.
Schmitt-Graff A, Kruger S, Bochard F, et al. Modulation of alpha smooth muscle actin and desmin expression in perisinusoidal cells of normal and diseased human livers. Am J Pathol 1991;138:1233–1242.
Clouston AD, Powell EE, Walsh MJ, et al. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology 2005;41:809–818.
Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 2004;39:1739–1745.
Castillo J, Goñi S, Latasa MU, et al. Amphiregulin induces the alternative splicing of p73 into its oncogenic isoform DeltaEx2p73 in human hepatocellular tumors. Gastroenterology 2009;137:1805–1815.
Andreakos E, Foxwell B, Feldmann M . Is targeting toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunol Rev 2004;202:250–265.
Guo J, Friedman SL . Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair 2010;3:21.
Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 2007;13:1324–1332.
Guo J, Loke J, Zheng F, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of toll-like receptor 4 to hepatic stellate cell responses. Hepatology 2009;49:960–968.
Gaudio E, Carpino G, Cardinale V, et al. New insights into liver stem cells. Dig Liver Dis 2009;41:455–462.
Sasatomi K, Noguchi K, Sakisaka S, et al. Abnormal accumulation of endotoxin in biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol 1998;29:409–416.
Glaser SS, Gaudio E, Miller T, et al. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med 2009;11:e7.
Lowes KN, Brennan BA, Yeoh GC, et al. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol 1999;154:537–541.
Grappone C, Pinzani M, Parola M, et al. Expression of platelet-derived growth factor in newly formed cholangiocytes during experimental biliary fibrosis in rats. J Hepatol 1999;31:100–109.
Sedlaczek N, Jia JD, Bauer M, et al. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol 2001;158:1239–1244.
Yasoshima M, Kono N, Sugawara H, et al. Increased expression of interleukin-6 and tumor necrosis factor-alpha in pathologic biliary epithelial cells: in situ and culture study. Lab Invest 1998;78:89–100.
Park J, Gores GJ, Patel T . Lipopolysaccharide induces cholangiocyte proliferation via an interleukin-6-mediated activation of p44/p42 mitogen-activated protein kinase. Hepatology 1999;29:1037–1043.
Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 2006;24:801–812.
Pevsner-Fischer M, Morad V, Cohen-Sfady M, et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 2007;109:1422–1432.
Lee SH, Hong B, Sharabi A, et al. Embryonic stem cells and mammary luminal progenitors directly sense and respond to microbial products. Stem Cells 2009;27:1604–1615.
Acknowledgements
We thank Alessandra Micera for her kind technical assistance with confocal microscopy. This study has been partly supported by the ‘Alberto Sordi’ Foundation, Rome, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Vespasiani-Gentilucci, U., Carotti, S., Onetti-Muda, A. et al. Toll-like receptor-4 expression by hepatic progenitor cells and biliary epithelial cells in HCV-related chronic liver disease. Mod Pathol 25, 576–589 (2012). https://doi.org/10.1038/modpathol.2011.197
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/modpathol.2011.197
Keywords
This article is cited by
-
Up-regulated TLR2 and TLR4 expressions in liver and spleen during acute murine T. gondii infection
Parasitology Research (2016)