Abstract
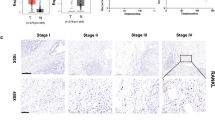
Bone metastases are a frequent complication of many cancers that result in severe disease burden and pain1,2,3. Since the late nineteenth century, it has been thought that the microenvironment of the local host tissue actively participates in the propensity of certain cancers to metastasize to specific organs, and that bone provides an especially fertile ‘soil’4. In the case of breast cancers, the local chemokine milieu is now emerging as an explanation for why these tumours preferentially metastasize to certain organs5. However, as the inhibition of chemokine receptors in vivo only partially blocks metastatic behaviour6, other factors must exist that regulate the preferential metastasis of breast cancer cells. Here we show that the cytokine RANKL (receptor activator of NF-κB ligand)7,8 triggers migration of human epithelial cancer cells and melanoma cells that express the receptor RANK. RANK is expressed on cancer cell lines and breast cancer cells in patients. In a mouse model of melanoma metastasis9, in vivo neutralization of RANKL by osteoprotegerin results in complete protection from paralysis and a marked reduction in tumour burden in bones but not in other organs. Our data show that local differentiation factors such as RANKL have an important role in cell migration and the tissue-specific metastatic behaviour of cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nature Rev. Cancer 2, 563–572 (2002)
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nature Rev. Cancer 2, 584–593 (2002)
Sloan, E. K. & Anderson, R. L. Genes involved in breast cancer metastasis to bone. Cell. Mol. Life Sci. 59, 1491–1502 (2002)
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1, 571–572 (1889)
Moore, M. A. The role of chemoattraction in cancer metastases. Bioessays 23, 674–676 (2001)
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001)
Kong, Y. Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999)
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998)
Arguello, F., Baggs, R. B. & Frantz, C. N. A murine model of experimental metastasis to bone and bone marrow. Cancer Res. 48, 6876–6881 (1988)
Anderson, D. M. et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390, 175–179 (1997)
Teitelbaum, S. L. Bone resorption by osteoclasts. Science 289, 1504–1508 (2000)
Fata, J. E. et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103, 41–50 (2000)
Verschueren, H. et al. Metastatic competence of BW5147 T-lymphoma cell lines is correlated with in vitro invasiveness, motility and F-actin content. J. Leukoc. Biol. 55, 552–556 (1994)
Liotta, L. A. & Kohn, E. C. The microenvironment of the tumour–host interface. Nature 411, 375–379 (2001)
Bakewell, S. J. et al. Platelet and osteoclast β3 integrins are critical for bone metastasis. Proc. Natl Acad. Sci. USA 100, 14205–14210 (2003)
Komarova, S. V., Pilkington, M. F., Weidema, A. F., Dixon, S. J. & Sims, S. M. RANK ligand-induced elevation of cytosolic Ca2+ accelerates nuclear translocation of nuclear factor κB in osteoclasts. J. Biol. Chem. 278, 8286–8293 (2003)
Sanchez-Sweatman, O. H., Lee, J., Orr, F. W. & Singh, G. Direct osteolysis induced by metastatic murine melanoma cells: role of matrix metalloproteinases. Eur. J. Cancer 33, 918–925 (1997)
Peyruchaud, O. et al. Early detection of bone metastases in a murine model of fluorescent human breast cancer cells: application to the use of the bisphosphonate zoledronic acid in the treatment of osteolytic lesions. J. Bone Miner. Res. 16, 2027–2034 (2001)
Guise, T. A. Molecular mechanisms of osteolytic bone metastases. Cancer 88 (Suppl.), 2892–2898 (2000)
Zhang, J. et al. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumour growth in the bone. J. Clin. Invest. 107, 1235–1244 (2001)
Morony, S. et al. Osteoprotegerin inhibits osteolysis and decreases skeletal tumour burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res. 61, 4432–4436 (2001)
Lelekakis, M. et al. A novel orthotopic model of breast cancer metastasis to bone. Clin. Exp. Metastasis 17, 163–170 (1999)
Acknowledgements
These studies were supported in part by grants from the Canadian Institutes of Health Research (CIHR/IMHA/TAS) and the Canadian Arthritis Network to S.M.S., S.J.D. and S.V.K. We thank W. Boyle, D. Lacey and C. Dunstan for providing rRANKL, RANKL-FITC and rOPG. J.M.P. is supported by the National Cancer Institute of Canada, IMBA, the Austrian National Bank and a European Union Marie Curie Excellence Grant. T.N. holds a European Union Marie Curie Mobility Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.M.P. has shares in Amgen Inc., and I.S. and S.M. work for Amgen Inc., which develops RANKL inhibitors as drugs.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1–7. (PPT 10235 kb)
Supplementary Notes
This file contains Supplementary Methods, Supplementary Figure Legends and additional references. (DOC 72 kb)
Rights and permissions
About this article
Cite this article
Jones, D., Nakashima, T., Sanchez, O. et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440, 692–696 (2006). https://doi.org/10.1038/nature04524
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/nature04524
This article is cited by
-
The immune regulatory function of B7-H3 in malignancy: spotlight on the IFN-STAT1 axis and regulation of tumor-associated macrophages
Immunologic Research (2024)
-
A Novel Frailty Grade Combined with Cachexia Index and Osteopenia in Esophagectomy
World Journal of Surgery (2023)
-
The effect of denosumab on disseminated tumor cells (DTCs) of breast cancer patients with neoadjuvant treatment: a GeparX translational substudy
Breast Cancer Research (2023)
-
Osteopenia is associated with inferior survival in patients undergoing partial hepatectomy for hepatocellular carcinoma
Scientific Reports (2022)
-
Identification of a binding site on soluble RANKL that can be targeted to inhibit soluble RANK-RANKL interactions and treat osteoporosis
Nature Communications (2022)