Abstract
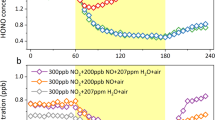
Nitrous acid is a significant photochemical precursor of the hydroxyl radical1,2,3,4,5,6,7,8,9,10,11,12,13, the key oxidant in the degradation of most air pollutants in the troposphere. The sources of nitrous acid in the troposphere, however, are still poorly understood. Recent atmospheric measurements7,10,11,12,13,14,15,16,17 revealed a strongly enhanced formation of nitrous acid during daytime via unknown mechanisms. Here we expose humic acid films to nitrogen dioxide in an irradiated tubular gas flow reactor and find that reduction of nitrogen dioxide on light-activated humic acids is an important source of gaseous nitrous acid. Our findings indicate that soil and other surfaces containing humic acid exhibit an organic surface photochemistry that produces reductive surface species, which react selectively with nitrogen dioxide. The observed rate of nitrous acid formation could explain the recently observed high daytime concentrations of nitrous acid in the boundary layer, the photolysis of which accounts for up to 60 per cent of the integrated hydroxyl radical source strengths3,6,7,8,9,10,11,12,13. We suggest that this photo-induced nitrous acid production on humic acid could have a potentially significant impact on the chemistry of the lowermost troposphere.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Perner, D. & Platt, U. Detection of nitrous acid in the atmosphere by differential optical-absorption. Geophys. Res. Lett. 6, 917–920 (1979)
Platt, U., Perner, D., Harris, G. W., Winer, A. M. & Pitts, J. N. Observations of nitrous-acid in an urban atmosphere by differential optical-absorption. Nature 285, 312–314 (1980)
Harrison, R. M., Peak, J. D. & Collins, G. M. Tropospheric cycle of nitrous acid. J. Geophys. Res. 101, 14429–14439 (1996)
Harris, G. W. et al. Observations of nitrous acid in the Los Angeles atmosphere and implications for predictions of ozone-precursor relationships. Environ. Sci. Technol. 16, 414–419 (1982)
Lammel, G. & Cape, J. N. Nitrous acid and nitrite in the atmosphere. Chem. Soc. Rev. 25, 361–369 (1996)
Alicke, B., Platt, U. & Stutz, J. Impact of nitrous acid photolysis on the total hydroxyl radical budget during the Limitation of Oxidant Production/Pianura Padana Produzione di Ozono study in Milan. J. Geophys. Res. 107, 8196, doi:10.1029/2000JD000075 (2002)
Zhou, X. L. et al. Summertime nitrous acid chemistry in the atmospheric boundary layer at a rural site in New York State. J. Geophys. Res. 107, 4590, doi:10.1029/2001JD001539 (2002)
Alicke, B. et al. OH formation by HONO photolysis during the BERLIOZ experiment. J. Geophys. Res. 108, 8247, doi:10.1029/2001JD000579 (2003)
Aumont, B., Chervier, F. & Laval, S. Contribution of HONO sources to the NOx/HOx/O3 chemistry in the polluted boundary layer. Atmos. Environ. 37, 487–498 (2003)
Vogel, B., Vogel, H., Kleffmann, J. & Kurtenbach, R. Measured and simulated vertical profiles of nitrous acid—Part II. Model simulations and indications for a photolytic source. Atmos. Environ. 37, 2957–2966 (2003)
Ren, X. R. et al. OH and HO2 chemistry in the urban atmosphere of New York City. Atmos. Environ. 37, 3639–3651 (2003)
Kleffmann, J. et al. Daytime formation of nitrous acid: A major source of OH radicals in a forest. Geophys. Res. Lett. 32, 05818, doi:10.1029/2005GL022524 (2005)
Acker, K. et al. Strong daytime production of OH from HNO2 at a rural mountain site. Geophys. Res. Lett. 33, 02809, doi:10.1029/2005GL024643 (2006)
Kleffmann, J. et al. Measured and simulated vertical profiles of nitrous acid—Part I: Field measurements. Atmos. Environ. 37, 2949–2955 (2003)
Zhou, X. L. et al. Nitric acid photolysis on surfaces in low-NOx environments: Significant atmospheric implications. Geophys. Res. Lett. 30, 2217, doi:10.1029/2003GL018620 (2003)
Staffelbach, T., Neftel, A. & Horowitz, L. W. Photochemical oxidant formation over southern Switzerland. 2. Model results. J. Geophys. Res. 102, 23363–23373 (1997)
Honrath, R. E. et al. Vertical fluxes of NOx, HONO, and HNO3 above the snowpack at Summit, Greenland. Atmos. Environ. 36, 2629–2640 (2002)
Finlayson-Pitts, B. J., Wingen, L. M., Sumner, A. L., Syomin, D. & Ramazan, K. A. The heterogeneous hydrolysis of NO2 in laboratory systems and in outdoor and indoor atmospheres: An integrated mechanism. Phys. Chem. Chem. Phys. 5, 223–242 (2003)
Krivacsy, Z. et al. Study of humic-like substances in fog and interstitial aerosol by size-exclusion chromatography and capillary electrophoresis. Atmos. Environ. 34, 4273–4281 (2000)
Janzen, H. H. Carbon cycling in earth systems - a soil science perspective. Agric. Ecosyst. Environ. 104, 399–417 (2004)
George, C., Strekowski, R. S., Kleffmann, J., Stemmler, K. & Ammann, M. Photoenhanced uptake of gaseous NO2 on solid organic compounds: A photochemical source of HONO? Faraday Discuss. 130, 195–210 (2005)
Ramazan, K. A., Syomin, D. & Finlayson-Pitts, B. J. The photochemical production of HONO during the heterogeneous hydrolysis of NO2 . Phys. Chem. Chem. Phys. 6, 3836–3843 (2004)
Blough, N. V. in The Sea surface and global change (eds Lyss, P. S. & Duce, P. A.) 383–425 (Cambridge University Press, Cambridge, 1997)
Ammann, M., Rössler, E., Strekowski, R. & George, C. Uptake of NO2 on aqueous solutions containing phenoxy type compounds - Implication for HONO formation in the atmosphere. Phys. Chem. Chem. Phys. 7, 2513–2518 (2005)
Venterea, R. T. & Rolston, D. E. Mechanisms and kinetics of nitric and nitrous oxide production during nitrification in agricultural soil. Glob. Change Biol. 6, 303–316 (2000)
Stevenson, F., Harrison, R. M., Wetselaar, R. & Leeper, R. A. Nitrosation of soil organic matter. 3. Nature of gases produced by reaction of nitrite with lignins, humic substances, and phenolic constituents under neutral and slightly acidic conditions. Soil Sci. Soc. Am. 34, 430–435 (1970)
Staffelbach, T. et al. Photochemical oxidant formation over southern Switzerland. 1. Results from summer 1994. J. Geophys. Res. 102, 23345–23362 (1997)
Atkinson, R. & Arey, J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review. Atmos. Environ. 37, 197–219 (2003)
Kleffmann, J., Heland, J., Kurtenbach, R., Lörzer, J. C. & Wiesen, P. A new instrument (LOPAP) for the detection of nitrous acid (HONO). Environ. Sci. Pollut. Res. 9, 48–54 (2002)
Zepp, R. G., Faust, B. C. & Hoigne, J. Hydroxyl radical formation in aqueous reactions of iron(II) with hydrogen peroxide—the photo-fenton reaction. Environ. Sci. Technol. 26, 313–319 (1992)
Acknowledgements
We thank Y. Abd El Aal, S. Canonica, M. Birrer, J. Dommen, A. Prêvot, L. Urech and I. Alxneit for discussions or technical support. K.S. thanks the Swiss National Science Foundation for support. C.G. acknowledges the grant by Primequal2 for the project SHONO and the CNRS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
This figure compares the photoreactivity of humic acids of different origin towards NO2. Shown are data for humic acids originating from peat, soil, or lignite-coal, which are all reactive towards NO2 and a reference experiment on uncoated glass surface under the same experimental conditions which shows no measurable reactivity. (PDF 38 kb)
Supplementary Figure 2
This figure shows the spectral irradiances of the three light sources used in the present study and compares them with the solar spectral irradiance at the earth surface. (PDF 54 kb)
Supplementary Figure 3
This figure compares the photo-formation of gaseous H2O2 and HONO on an Aldrich Humic Acid surface under UV-A irradiation. While only minor amounts of H2O2 are formed, a substantial amount of HONO is produced on this surface. (PDF 47 kb)
Rights and permissions
About this article
Cite this article
Stemmler, K., Ammann, M., Donders, C. et al. Photosensitized reduction of nitrogen dioxide on humic acid as a source of nitrous acid. Nature 440, 195–198 (2006). https://doi.org/10.1038/nature04603
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/nature04603
This article is cited by
-
Highly selective and efficient photocatalytic NO removal: Charge carrier kinetics and interface molecular process
Nano Research (2024)
-
Synthesizing evidence for the external cycling of NOx in high- to low-NOx atmospheres
Nature Communications (2023)
-
Formation of nitrogen-containing gas phase products from the heterogeneous (photo)reaction of NO2 with gallic acid
Communications Chemistry (2023)
-
Effects of drought and recovery on soil volatile organic compound fluxes in an experimental rainforest
Nature Communications (2023)
-
Atmospheric NOx oxidation as major sources for nitrous acid (HONO)
npj Climate and Atmospheric Science (2023)