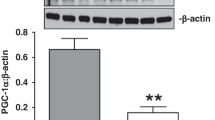
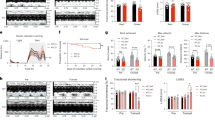
Abstract
Ischaemia of the heart, brain and limbs is a leading cause of morbidity and mortality worldwide. Hypoxia stimulates the secretion of vascular endothelial growth factor (VEGF) and other angiogenic factors, leading to neovascularization and protection against ischaemic injury1. Here we show that the transcriptional coactivator PGC-1α (peroxisome-proliferator-activated receptor-γ coactivator-1α), a potent metabolic sensor and regulator2, is induced by a lack of nutrients and oxygen, and PGC-1α powerfully regulates VEGF expression and angiogenesis in cultured muscle cells and skeletal muscle in vivo. PGC-1α-/- mice show a striking failure to reconstitute blood flow in a normal manner to the limb after an ischaemic insult, whereas transgenic expression of PGC-1α in skeletal muscle is protective. Surprisingly, the induction of VEGF by PGC-1α does not involve the canonical hypoxia response pathway and hypoxia inducible factor (HIF). Instead, PGC-1α coactivates the orphan nuclear receptor ERR-α (oestrogen-related receptor-α) on conserved binding sites found in the promoter and in a cluster within the first intron of the VEGF gene. Thus, PGC-1α and ERR-α, major regulators of mitochondrial function in response to exercise and other stimuli, also control a novel angiogenic pathway that delivers needed oxygen and substrates. PGC-1α may provide a novel therapeutic target for treating ischaemic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carmeliet, P. Angiogenesis in health and disease. Nature Med. 9, 653–660 (2003)
Lin, J., Handschin, C. & Spiegelman, B. M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1, 361–370 (2005)
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999)
St-Pierre, J. et al. Bioenergetic analysis of peroxisome proliferator-activated receptor γ coactivators 1α and 1β (PGC-1α and PGC-1β) in muscle cells. J. Biol. Chem. 278, 26597–26603 (2003)
Semenza, G. L. Angiogenesis in ischemic and neoplastic disorders. Annu. Rev. Med. 54, 17–28 (2003)
Jain, R. K. Molecular regulation of vessel maturation. Nature Med. 9, 685–693 (2003)
Lin, J. et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 (2002)
Couffinhal, T. et al. Mouse model of angiogenesis. Am. J. Pathol. 152, 1667–1679 (1998)
Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nature Med. 9, 669–676 (2003)
Wood, S. M., Gleadle, J. M., Pugh, C. W., Hankinson, O. & Ratcliffe, P. J. The role of the aryl hydrocarbon receptor nuclear translocator (ARNT) in hypoxic induction of gene expression. Studies in ARNT-deficient cells. J. Biol. Chem. 271, 15117–15123 (1996)
Kelly, D. P. & Scarpulla, R. C. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 18, 357–368 (2004)
Kressler, D., Schreiber, S. N., Knutti, D. & Kralli, A. The PGC-1-related protein PERC is a selective coactivator of estrogen receptor alpha. J. Biol. Chem. 277, 13918–13925 (2002)
Schreiber, S. N., Knutti, D., Brogli, K., Uhlmann, T. & Kralli, A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor α (ERRα). J. Biol. Chem. 278, 9013–9018 (2003)
Huss, J. M., Kopp, R. P. & Kelly, D. P. Peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-α and -γ. Identification of novel leucine-rich interaction motif within PGC-1α. J. Biol. Chem. 277, 40265–40274 (2002)
Huss, J. M., Torra, I. P., Staels, B., Giguere, V. & Kelly, D. P. Estrogen-related receptor α directs peroxisome proliferator-activated receptor α signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol. Cell. Biol. 24, 9079–9091 (2004)
Mootha, V. K. et al. Errα and Gabpa/b specify PGC-1α-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc. Natl Acad. Sci. USA 101, 6570–6575 (2004)
Sladek, R., Bader, J. A. & Giguere, V. The orphan nuclear receptor estrogen-related receptor α is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol. Cell. Biol. 17, 5400–5409 (1997)
Shaw, R. J. Glucose metabolism and cancer. Curr. Opin. Cell Biol. 18, 598–608 (2006)
Carmeliet, P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nature Med. 6, 1102–1103 (2000)
Henry, T. D. et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation 107, 1359–1365 (2003)
Pajusola, K. et al. Stabilized HIF-1α is superior to VEGF for angiogenesis in skeletal muscle via adeno-associated virus gene transfer. FASEB J. 19, 1365–1367 (2005)
Arany, Z. et al. The transcriptional coactivator PGC-1β drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 5, 35–46 (2007)
Megeney, L. A., Kablar, B., Garrett, K., Anderson, J. E. & Rudnicki, M. A. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 10, 1173–1183 (1996)
Acknowledgements
We thank E. Smith for assistance with graphics. This work was supported by grants from the National Institutes of Health (Z.A. and B.M.S.), the Wenner–Gren Foundation (J.L.R.) and the Leducq Foundation (A.R. and B.M.S.).
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Figures
The file contains Supplementary Figures S1-S22 with Legends. (PDF 1059 kb)
Rights and permissions
About this article
Cite this article
Arany, Z., Foo, SY., Ma, Y. et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 451, 1008–1012 (2008). https://doi.org/10.1038/nature06613
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/nature06613
This article is cited by
-
ERRα: unraveling its role as a key player in cell migration
Oncogene (2024)
-
NADH elevation during chronic hypoxia leads to VHL-mediated HIF-1α degradation via SIRT1 inhibition
Cell & Bioscience (2023)
-
Angiogenesis after ischemic stroke
Acta Pharmacologica Sinica (2023)
-
Exercise metabolism and adaptation in skeletal muscle
Nature Reviews Molecular Cell Biology (2023)
-
Biology and therapeutic targeting of vascular endothelial growth factor A
Nature Reviews Molecular Cell Biology (2023)