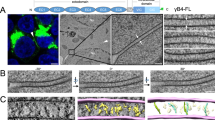
Abstract
Dendritic arborizations of many neurons are patterned by a process called self-avoidance, in which branches arising from a single neuron repel each other1,2,3,4,5,6,7. By minimizing gaps and overlaps within the arborization, self-avoidance facilitates complete coverage of a neuron’s territory by its neurites1,2,3. Remarkably, some neurons that display self-avoidance interact freely with other neurons of the same subtype, implying that they discriminate self from non-self. Here we demonstrate roles for the clustered protocadherins (Pcdhs) in dendritic self-avoidance and self/non-self discrimination. The Pcdh locus encodes 58 related cadherin-like transmembrane proteins, at least some of which exhibit isoform-specific homophilic adhesion in heterologous cells and are expressed stochastically and combinatorially in single neurons7,8,9,10,11. Deletion of all 22 Pcdh genes in the mouse γ-subcluster (Pcdhg genes) disrupts self-avoidance of dendrites in retinal starburst amacrine cells (SACs) and cerebellar Purkinje cells. Further genetic analysis of SACs showed that Pcdhg proteins act cell-autonomously during development, and that replacement of the 22 Pcdhg proteins with a single isoform restores self-avoidance. Moreover, expression of the same single isoform in all SACs decreases interactions among dendrites of neighbouring SACs (heteroneuronal interactions). These results suggest that homophilic Pcdhg interactions between sibling neurites (isoneuronal interactions) generate a repulsive signal that leads to self-avoidance. In this model, heteroneuronal interactions are normally permitted because dendrites seldom encounter a matched set of Pcdhg proteins unless they emanate from the same soma. In many respects, our results mirror those reported for Dscam1 (Down syndrome cell adhesion molecule) in Drosophila: this complex gene encodes thousands of recognition molecules that exhibit stochastic expression and isoform-specific interactions, and mediate both self-avoidance and self/non-self discrimination4,5,6,7,12,13,14,15. Thus, although insect Dscam and vertebrate Pcdh proteins share no sequence homology, they seem to underlie similar strategies for endowing neurons with distinct molecular identities and patterning their arborizations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kramer, A. P. & Kuwada, J. Y. Formation of the receptive fields of leech mechanosensory neurons during embryonic development. J. Neurosci. 3, 2474–2486 (1983)
Montague, P. R. & Friedlander, M. J. Expression of an intrinsic growth strategy by mammalian retinal neurons. Proc. Natl Acad. Sci. USA 86, 7223–7227 (1989)
Grueber, W. B. & Sagasti, A. Self-avoidance and tiling: mechanisms of dendrite and axon spacing. Cold Spring Harb. Perspect. Biol. 2, a001750 (2010)
Matthews, B. J. et al. Dendrite self-avoidance is controlled by Dscam. Cell 129, 593–604 (2007)
Soba, P. et al. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron 54, 403–416 (2007)
Hughes, M. E. et al. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron 54, 417–427 (2007)
Zipursky, S. L. & Sanes, J. R. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell 143, 343–353 (2010)
Wu, Q. & Maniatis, T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell 97, 779–790 (1999)
Kohmura, N. et al. Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron 20, 1137–1151 (1998)
Kaneko, R. et al. Allelic gene regulation of Pcdh-α and Pcdh-γ clusters involving both monoallelic and biallelic expression in single Purkinje cells. J. Biol. Chem. 281, 30551–30560 (2006)
Schreiner, D. & Weiner, J. A. Combinatorial homophilic interaction between γ-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proc. Natl Acad. Sci. USA 107, 14893–14898 (2010)
Zhan, X. L. et al. Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron 43, 673–686 (2004)
Wojtowicz, W. M., Flanagan, J. J., Millard, S. S., Zipursky, S. L. & Clemens, J. C. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell 118, 619–633 (2004)
Neves, G., Zucker, J., Daly, M. & Chess, A. Stochastic yet biased expression of multiple Dscam splice variants by individual cells. Nature Genet. 36, 240–246 (2004)
Hattori, D. et al. Robust discrimination between self and non-self neurites requires thousands of Dscam1 isoforms. Nature 461, 644–648 (2009)
Yamagata, M. & Sanes, J. R. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature 451, 465–469 (2008)
Fuerst, P. G., Koizumi, A., Masland, R. H. & Burgess, R. W. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature 451, 470–474 (2008)
Fuerst, P. G. et al. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron 64, 484–497 (2009)
Sanes, J. R. & Zipursky, S. L. Design principles of insect and vertebrate visual systems. Neuron 66, 15–36 (2010)
Wang, X. et al. Gamma protocadherins are required for survival of spinal interneurons. Neuron 36, 843–854 (2002)
Prasad, T., Wang, X., Gray, P. A. & Weiner, J. A. A differential developmental pattern of spinal interneuron apoptosis during synaptogenesis: insights from genetic analyses of the protocadherin-γ gene cluster. Development 135, 4153–4164 (2008)
Lefebvre, J. L., Zhang, Y., Meister, M., Wang, X. & Sanes, J. R. γ-Protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development 135, 4141–4151 (2008)
Weiner, J. A., Wang, X., Tapia, J. C. & Sanes, J. R. Gamma protocadherins are required for synaptic development in the spinal cord. Proc. Natl Acad. Sci. USA 102, 8–14 (2005)
Stacy, R. C. & Wong, R. O. Developmental relationship between cholinergic amacrine cell processes and ganglion cell dendrites of the mouse retina. J. Comp. Neurol. 456, 154–166 (2003)
Lee, S. & Zhou, Z. J. The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron 51, 787–799 (2006)
White, F. A., Keller-Peck, C. R., Knudson, C. M., Korsmeyer, S. J. & Snider, W. D. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J. Neurosci. 18, 1428–1439 (1998)
Jelinek, H. F. & Fernandez, E. Neurons and fractals: how reliable and useful are calculations of fractal dimensions? J. Neurosci. Methods 81, 9–18 (1998)
Chen, W. V. et al. Functional significance of isoform diversification in the protocadherin gamma gene cluster. Neuron (in the press)
Wang, J. et al. Transmembrane/juxtamembrane domain-dependent Dscam distribution and function during mushroom body neuronal morphogenesis. Neuron 43, 663–672 (2004)
Kaneko, M. et al. Remodeling of monoplanar Purkinje cell dendrites during cerebellar circuit formation. PLoS ONE 6, e20108 (2011)
Rowan, S. & Cepko, C. L. Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev. Biol. 271, 388–402 (2004)
Furuta, Y., Lagutin, O., Hogan, B. L. & Oliver, G. C. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis 26, 130–132 (2000)
Knudson, C. M., Tung, K. S., Tourtellotte, W. G., Brown, G. A. & Korsmeyer, S. J. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270, 96–99 (1995)
Zhang, X. M. et al. Highly restricted expression of Cre recombinase in cerebellar Purkinje cells. Genesis 40, 45–51 (2004)
Rossi, J. et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 13, 195–204 (2011)
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neurosci. 13, 133–140 (2010)
Kay, J. N. et al. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J. Neurosci. 31, 7753–7762 (2011)
Srinivas, S. et al. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev. Genet. 24, 241–251 (1999)
Yamagata, M. &. Sanes, J. R. Transgenic strategy for identifying synaptic connections in mice by fluorescence complementation (GRASP). Front. Mol. Neurosci. 5, 18 (2012)
Farley, F. W., Soriano, P., Steffen, L. S. & Dymecki, S. M. Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28, 106–110 (2000)
Kuchibhotla, K. V. et al. Aβ plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59, 214–225 (2008)
Hong, Y. K., Kim, I. J. & Sanes, J. R. Stereotyped axonal arbors of retinal ganglion cell subsets in the mouse superior colliculus. J. Comp. Neurol. 519, 1691–1711 (2011)
Meyer-Franke, A., Kaplan, M. R., Pfrieger, F. W. & Barres, B. A. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron 15, 805–819 (1995)
Chen, Y. et al. NS21: re-defined and modified supplement B27 for neuronal cultures. J. Neurosci. Methods 171, 239–247 (2008)
Whitney, I. E., Keeley, P. W., Raven, M. A. & Reese, B. E. Spatial patterning of cholinergic amacrine cells in the mouse retina. J. Comp. Neurol. 508, 1–12 (2008)
Rodieck, R. W. The density recovery profile: a method for the analysis of points in the plane applicable to retinal studies. Vis. Neurosci. 6, 95–111 (1991)
Montague, P. R. & Friedlander, M. J. Morphogenesis and territorial coverage by isolated mammalian retinal ganglion cells. J. Neurosci. 11, 1440–1457 (1991)
Smith, T. G., Jr, Lange, G. D. & Marks, W. B. Fractal methods and results in cellular morphology—dimensions, lacunarity and multifractals. J. Neurosci. Methods 69, 123–136 (1996)
Wässle, H., Peichl, L. & Boycott, B. B. Dendritic territories of cat retinal ganglion cells. Nature 292, 344–345 (1981)
Kay, J. N., Voinescu, P. E., Chu, M. W. & Sanes, J. R. Neurod6 expression defines new retinal amacrine cell subtypes and regulates their fate. Nature Neurosci. 14, 965–972 (2011)
Acknowledgements
We thank members of our laboratory for providing advice and reagents, including D. Cai and K. Cohen (rAAV), I.-J. Kim (fstl4-line 1 mice) and M. Yamagata for modified Rosa-CAG targeting vector. We also thank B. Stevens (Children’s Hospital) for advice on culture methods. This work was supported by grants from NIH to J.R.S. (R01NS029169 and R01EY022073) and T.M. (R01NS043915) and NARSAD Young Investigator Award to J.L.L.
Author information
Authors and Affiliations
Contributions
J.L.L., D.K. and J.R.S. designed experiments and prepared the manuscript. J.L.L. and D.K. performed experiments and data analysis. J.R.S. supervised the project. W.V.C. and T.M. generated Pcdhgtako and Pcdhgtcko mice. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 and Supplementary Table 1. (PDF 4848 kb)
Rights and permissions
About this article
Cite this article
Lefebvre, J., Kostadinov, D., Chen, W. et al. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature 488, 517–521 (2012). https://doi.org/10.1038/nature11305
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nature11305
This article is cited by
-
CTCF loss induces giant lamellar bodies in Purkinje cell dendrites
Acta Neuropathologica Communications (2022)
-
Patterned cPCDH expression regulates the fine organization of the neocortex
Nature (2022)
-
Mathematical models of neuronal growth
Biomechanics and Modeling in Mechanobiology (2022)
-
Development of FRET-based indicators for visualizing homophilic trans interaction of a clustered protocadherin
Scientific Reports (2021)
-
Theory of branching morphogenesis by local interactions and global guidance
Nature Communications (2021)