Abstract
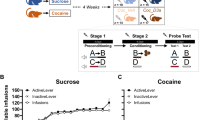
Loss of control over harmful drug seeking is one of the most intractable aspects of addiction, as human substance abusers continue to pursue drugs despite incurring significant negative consequences1. Human studies have suggested that deficits in prefrontal cortical function and consequential loss of inhibitory control2,3,4 could be crucial in promoting compulsive drug use. However, it remains unknown whether chronic drug use compromises cortical activity and, equally important, whether this deficit promotes compulsive cocaine seeking. Here we use a rat model of compulsive drug seeking5,6,7,8 in which cocaine seeking persists in a subgroup of rats despite delivery of noxious foot shocks. We show that prolonged cocaine self-administration decreases ex vivo intrinsic excitability of deep-layer pyramidal neurons in the prelimbic cortex, which was significantly more pronounced in compulsive drug-seeking animals. Furthermore, compensating for hypoactive prelimbic cortex neurons with in vivo optogenetic prelimbic cortex stimulation significantly prevented compulsive cocaine seeking, whereas optogenetic prelimbic cortex inhibition significantly increased compulsive cocaine seeking. Our results show a marked reduction in prelimbic cortex excitability in compulsive cocaine-seeking rats, and that in vivo optogenetic prelimbic cortex stimulation decreased compulsive drug-seeking behaviours. Thus, targeted stimulation of the prefrontal cortex could serve as a promising therapy for treating compulsive drug use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th edn. (2000)
Naqvi, N. H. & Bechara, A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct. Funct. 214, 435–450 (2010)
Goldstein, R. Z. & Volkow, N. D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Rev. Neurosci. 12, 652–669 (2011)
Jentsch, J. D. & Taylor, J. R. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacol. 146, 373–390 (1999)
Pelloux, Y., Everitt, B. J. & Dickinson, A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacol. 194, 127–137 (2007)
Belin, D., Mar, A. C., Dalley, J. W., Robbins, T. W. & Everitt, B. J. High impulsivity predicts the switch to compulsive cocaine-taking. Science 320, 1352–1355 (2008)
Vanderschuren, L. J. & Everitt, B. J. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305, 1017–1019 (2004)
Deroche-Gamonet, V., Belin, D. & Piazza, P. V. Evidence for addiction-like behavior in the rat. Science 305, 1014–1017 (2004)
Uylings, H. B. M., Groenewegen, J. J. & Kolb, B. Do rats have a prefrontal cortex? Behav. Brain Res. 146, 3–17 (2003)
Farovik, A., Dupont, L. M., Arce, M. & Eichenbaum, H. Medial prefrontal cortex supports recollection, but not familiarity, in the rat. J. Neurosci. 28, 13428–13434 (2008)
Grégoire, S., Rivalan, M., Le Moine, C. & Dellu-Hagedorn, F. The synergy of working memory and inhibitory control: behavioral, pharmacological and neural functional evidences. Neurobiol. Learn. Mem. 97, 202–212 (2012)
Hare, T. A., Camerer, C. F. & Rangel, A. Self-control in decision-making involves modulation of the vmPFC valuations. Science 324, 646–648 (2009)
Balleine, B. W. & O’Doherty, J. P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacol. 35, 48–69 (2009)
Jonkman, S., Mar, A. C., Dickinson, A., Robbins, T. W. & Everitt, B. J. The rat prelimbic cortex mediates inhibitory response control but not the consolidation of instrumental learning. Behav. Neurosci. 123, 875–885 (2009)
Peters, J., Kalivas, P. W. & Quirk, G. J. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn. Mem. 16, 279–288 (2009)
Krishnan, V. et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404 (2007)
Beck, H. & Yaari, Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nature Rev. Neurosci. 9, 357–369 (2008)
Yang, C. R., Seamans, J. K. & Gorelova, N. Electrophysiological and morphological properties of layers V–VI principal pyramidal cells in rat prefrontal cortex in vitro. J. Neurosci. 16, 1904–1921 (1996)
Ghazizadeh, A., Ambroggi, F., Odean, N. & Fields, H. L. Prefrontal cortex mediates extinction of responding by two distinct neural mechanisms in accumbens shell. J. Neurosci. 32, 726–737 (2012)
Sesack, S. R., Deutch, A. Y., Roth, R. H. & Bunney, B. S. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 290, 213–242 (1989)
Zhang, F., Wang, L.-P., Boyden, E. S. & Deisseroth, K. Channelrhodopsin-2 and optical control of excitable cells. Nature Methods 3, 785–792 (2006)
Witten, I. B. et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72, 721–733 (2011)
Moussawi, K. et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nature Neurosci. 12, 182–189 (2009)
Kasanetz, F. et al. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol. Psychiatry http://dx.doi.org/10.1038/mp.2012.59 (15 May 2012)
Tiffany, S. T. & Conklin, C. A. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction 95 (suppl. 2). S145–S153 (2000)
Martina, M., Schultz, J. H., Ehmke, H., Monyer, H. & Jonas, P. Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J. Neurosci. 18, 8111–8125 (1998)
Taverna, S., Tkatch, T., Metz, A. E. & Martina, M. Differential expression of TASK channels between horizontal interneurons and pyramidal cells of rat hippocampus. J. Neurosci. 25, 9162–9170 (2005)
Chen, B. T. et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron 59, 288–297 (2008)
Knackstedt, L. A. & Kalivas, P. W. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J. Pharmacol. Exp. Ther. 322, 1103–1109 (2007)
Stuber, G. D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011)
Acknowledgements
We thank S. Kourrich and Y. Shaham for careful reading of the manuscript. We also thank K. Deisseroth for providing the ChR2 and eNpHR3.0 vectors. This study was supported by funds from the NIDA/IRP and the State of California through the University of California at San Francisco.
Author information
Authors and Affiliations
Contributions
B.T.C., H.-J.Y., F.W.H. and A.B. designed, discussed and planned all experiments. B.T.C., H.-J.Y., I.K.-Y., C.H. and S.L.C. performed experiments. B.T.C. and C.H. analysed data. B.T.C., F.W.H. and A.B. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12, Supplementary Table 1 and Supplementary References. (PDF 795 kb)
Rights and permissions
About this article
Cite this article
Chen, B., Yau, HJ., Hatch, C. et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496, 359–362 (2013). https://doi.org/10.1038/nature12024
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nature12024
This article is cited by
-
Astrocytes modulate cerebral blood flow and neuronal response to cocaine in prefrontal cortex
Molecular Psychiatry (2024)
-
Incubation of methamphetamine craving in punishment-resistant individuals is associated with activation of specific gene networks in the rat dorsal striatum
Molecular Psychiatry (2024)
-
Current perspectives on brain circuits involved in food addiction-like behaviors
Journal of Neural Transmission (2024)
-
Pathways to the persistence of drug use despite its adverse consequences
Molecular Psychiatry (2023)
-
Cell-type specific synaptic plasticity in dorsal striatum is associated with punishment-resistance compulsive-like cocaine self-administration in mice
Neuropsychopharmacology (2023)


Peter Cary
On behalf of Yi-Yuan Tang:
Brief meditation training induces smoking reduction
Yi-Yuan Tang a*, Rongxiang Tang b, Michael Posner c
a Department of Psychology & Texas Tech Neuroimaging Institute, Texas Tech University, TX 79409, USA.
b Department of Psychology, The University of Texas at Austin, TX 78705, USA
c Department of Psychology, University of Oregon, OR 97403, USA.
*Correspondence to: yiyuan.tang@ttu.edu mposner@uoregon.edu; rongxiangtang@yahoo.com.
Individuals at risk for substance abuse have deficits in self-control. Dysfunction of the prefrontal cortex (PFC) and anterior cingulate cortex (ACC) play a key role in addiction.
Using a rat model, Chen et al. reported a marked deficit in control of drug-seeking associated with reduced prelimbic excitability. Prelimbic stimulation decreased drug-seeking behaviors, indicating a possible therapy for treating compulsive drug use1. Is there any non-pharmaceutical intervention for human addiction targeting the PFC and ACC found reduced in rats? There is evidence that meditation ameliorates negative outcomes from deficits in self-control. In a series of randomized controlled trials, we found that a form of meditation, the integrative body-mind training (IBMT), reduced stress, increased positive emotion, and improved attention and self-control after a few hours of practice compared to the same amount of relaxation training (RT). Moreover, these positive changes were accompanied by increased ACC and parasympathetic activity associated with a brain state of increased self-control2. In our recent work3, we tested whether improved self-control through IBMT would reduce craving and smoking. We advertised for volunteers wishing to reduce stress and improve performance. Among those who responded, we randomly assigned both smokers and nonsmokers to IBMT or RT groups. Both groups received 2-wk training for a total of 5 hours. Our results showed 5 hours of IBMT produced a significant reduction in smoking of 60%, while no reduction was found in RT. Meanwhile, 5 hours of IBMT also significantly reduced craving compared to RT. Resting state brain imaging showed significantly increased activity in meditation group in the ACC and PFC, brain areas related to self-control. These results suggest brief meditation training improves self-control capacity and reduces smoking and craving.
Acknowledgments.
This work was supported by R21DA030066, 973 Program 2012CB518200 and the Office of Naval Research.
References
1. Chen et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 496, 359-62 (2013).
2. Tang YY, et al. Short-term meditation training improves attention and self-regulation. Proc. Natl. Acad. Sci. U.S.A 104, 17152?17156 (2007).
3. Tang, Y.Y., Tang, R., & Posner M.I. Brief meditation training induces smoking reduction. Proc Natl Acad Sci U S A., published ahead of print August 5, 2013, doi:10.1073/pnas.1311887110