Abstract
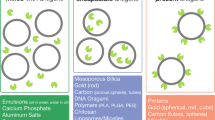
Antigen targeting1,2,3,4,5 and adjuvancy schemes6,7 that respectively facilitate delivery of antigen to dendritic cells and elicit their activation have been explored in vaccine development. Here we investigate whether nanoparticles can be used as a vaccine platform by targeting lymph node–residing dendritic cells via interstitial flow and activating these cells by in situ complement activation. After intradermal injection, interstitial flow transported ultra-small nanoparticles (25 nm) highly efficiently into lymphatic capillaries and their draining lymph nodes, targeting half of the lymph node–residing dendritic cells, whereas 100-nm nanoparticles were only 10% as efficient. The surface chemistry of these nanoparticles activated the complement cascade, generating a danger signal in situ and potently activating dendritic cells. Using nanoparticles conjugated to the model antigen ovalbumin, we demonstrate generation of humoral and cellular immunity in mice in a size- and complement-dependent manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bonifaz, L.C. et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199, 815–824 (2004).
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007).
Kwon, Y.J., James, E., Shastri, N. & Frechet, J.M. In vivo targeting of dendritic cells for activation of cellular immunity using vaccine carriers based on pH-responsive microparticles. Proc. Natl. Acad. Sci. USA 102, 18264–18268 (2005).
Trumpfheller, C. et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J. Exp. Med. 203, 607–617 (2006).
Wang, C. et al. Molecularly engineered poly(ortho ester) microspheres for enhanced delivery of DNA vaccines. Nat. Mater. 3, 190–196 (2004).
O'Hagan, D.T. & Valiante, N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2, 727–735 (2003).
Ulevitch, R.J. Therapeutics targeting the innate immune system. Nat. Rev. Immunol. 4, 512–520 (2004).
Rehor, A., Hubbell, J.A. & Tirelli, N. Oxidation-sensitive polymeric nanoparticles. Langmuir 21, 411–417 (2005).
Wilson, N.S. et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood 102, 2187–2194 (2003).
Reddy, S.T., Swartz, M.A. & Hubbell, J.A. Targeting dendritic cells with biomaterials: developing the next generation of vaccines. Trends Immunol. 27, 573–579 (2006).
Randolph, G.J., Angeli, V. & Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5, 617–628 (2005).
Pack, D.W. Timing is everything. Nat. Mater. 3, 133–134 (2004).
Little, S.R. et al. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc. Natl. Acad. Sci. USA 101, 9534–9539 (2004).
Storni, T. et al. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J. Immunol. 172, 1777–1785 (2004).
Fifis, T. et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 173, 3148–3154 (2004).
van Broekhoven, C.L., Parish, C.R., Demangel, C., Britton, W.J. & Altin, J.G. Targeting dendritic cells with antigen-containing liposomes: a highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res. 64, 4357–4365 (2004).
Swartz, M.A. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 50, 3–20 (2001).
Swartz, M.A. & Fleury, M.E. Interstitial flow and its effects in soft tissues. Annu. Rev. Biomed. Eng. 9, 229–256 (2007).
Reddy, S.T., Rehor, A., Schmoekel, H.G., Hubbell, J.A. & Swartz, M.A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 112, 26–34 (2006).
Nishioka, Y. & Yoshino, H. Lymphatic targeting with nanoparticulate system. Adv. Drug Deliv. Rev. 47, 55–64 (2001).
Reddy, S.T., Berk, D.A., Jain, R.K. & Swartz, M.A. A sensitive in vivo model for quantifying interstitial convective transport of injected macromolecules and nanoparticles. J. Appl. Physiol. 101, 1162–1169 (2006).
Boscardin, S.B. et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J. Exp. Med. 203, 599–606 (2006).
Carroll, M.C. The complement system in regulation of adaptive immunity. Nat. Immunol. 5, 981–986 (2004).
Dempsey, P.W., Allison, M.E., Akkaraju, S., Goodnow, C.C. & Fearon, D.T. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271, 348–350 (1996).
Kemper, C. & Atkinson, J.P. T-cell regulation: with complements from innate immunity. Nat. Rev. Immunol. 7, 9–18 (2007).
Kopf, M., Abel, B., Gallimore, A., Carroll, M. & Bachmann, M.F. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 8, 373–378 (2002).
Gadjeva, M. et al. The covalent binding reaction of complement component C3. J. Immunol. 161, 985–990 (1998).
Tang, L., Liu, L. & Elwing, H.B. Complement activation and inflammation triggered by model biomaterial surfaces. J. Biomed. Mater. Res. 41, 333–340 (1998).
Nilsson, B., Ekdahl, K.N., Mollnes, T.E. & Lambris, J.D. The role of complement in biomaterial-induced inflammation. Mol. Immunol. 44, 82–94 (2007).
Kidane, A. & Park, K. Complement activation by PEO-grafted glass surfaces. J. Biomed. Mater. Res. 48, 640–647 (1999).
Acknowledgements
We thank M. Pasquier, V. Borel, V. Garea for valuable technical assistance; J.M. Rutkowski for scientific discussions; J.B. Dixon for MATLAB programming. Project funded by the Competence Centre for Materials Science and Technology (CCMX) of the ETH-Board, Switzerland (to M.A.S. and J.A.H.).
Author information
Authors and Affiliations
Contributions
S.T.R. designed and performed the research, analyzed the data and wrote the manuscript; A.J.v.d.V. synthesized materials and analyzed synthetic data; E.S. developed methods on T-cell activation and analyzed the data; V.A. performed the research on T-cell adoptive transfer, analyzed the data and provided useful discussion on interpretation; G.J.R. analyzed the data and provided useful discussion on interpretation; C.P.O. synthesized materials and analyzed synthetic data; L.K.L. developed methods on T-cell activation and antibody titer measurement; M.A.S. designed the research, analyzed the data and wrote the manuscript; J.A.H. designed the research, analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The Ecole Polytechnique Fédérale de Lausanne has filed a patent application on technology related to this manuscript, and S.T.R., A.J.v.d.V, M.A.S. and J.A.H. are named as inventors on this application.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4; Supplementary Methods (PDF 428 kb)
Rights and permissions
About this article
Cite this article
Reddy, S., van der Vlies, A., Simeoni, E. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol 25, 1159–1164 (2007). https://doi.org/10.1038/nbt1332
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nbt1332
This article is cited by
-
Multifunctional nanocarriers for targeted drug delivery and diagnostic applications of lymph nodes metastasis: a review of recent trends and future perspectives
Journal of Nanobiotechnology (2023)
-
Senescent cancer cell-derived nanovesicle as a personalized therapeutic cancer vaccine
Experimental & Molecular Medicine (2023)
-
Application of Nano-Delivery Systems in Lymph Nodes for Tumor Immunotherapy
Nano-Micro Letters (2023)
-
Hyaluronic acid-antigens conjugates trigger potent immune response in both prophylactic and therapeutic immunization in a melanoma model
Drug Delivery and Translational Research (2023)
-
A multiepitope vaccine encoding four Eimeria epitopes with PLGA nanospheres: a novel vaccine candidate against coccidiosis in laying chickens
Veterinary Research (2022)