Abstract
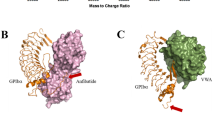
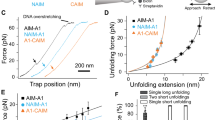
The A1 domain of von Willebrand factor (VWF-A1) plays a crucial role in hemostasis and thrombosis by initiating platelet adhesion at sites of arterial injury through interactions with the platelet receptor glycoprotein Ib alpha (GPIbα)1,2,3,4,5. Here we report that murine VWF-A1 supports limited binding of human platelets. However, atomic models of GPIbα–VWF-A1 complexes identified an electrostatic 'hot-spot' that, when mutated in murine VWF-A1, switches its binding specificity from mouse to human GPIbα. Furthermore, mice expressing this mutant VWF-A1 display a bleeding phenotype that can be corrected by infusion of human platelets. Mechanistically, human platelets correct the phenotype by forming occlusive thrombi, an event that can be abrogated by blockade of GPIbα or by the preadministration of inhibitors of platelet activation or adhesion (clopidogrel (Plavix) and abciximab (ReoPro), respectively). Thus, by modifying a protein interface, we have generated a potential biological platform for preclinical screening of antithrombotics that specifically target human platelets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Cruz, M.A., Diacovo, T.G., Emsley, J., Liddington, R. & Handin, R.I. Mapping the glycoprotein Ib-binding site in the von Willebrand factor A1 domain. J. Biol. Chem. 275, 19098–19105 (2000).
Sugimoto, M. et al. Functional modulation of the isolated glycoprotein Ib binding domain of von Willebrand factor expressed in Escherichia coli. Biochemistry 30, 5202–5209 (1991).
Pietu, G. et al. Production in Escherichia coli of a biologically active subfragment of von Willebrand factor corresponding to the platelet glycoprotein Ib, collagen and heparin binding domains. Biochem. Biophys. Res. Commun. 164, 1339–1347 (1989).
Savage, B., Saldivar, E. & Ruggeri, Z.M. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 84, 289–297 (1996).
Doggett, T.A. et al. Selectin-like kinetics and biomechanics promote rapid platelet adhesion in flow: the GPIb(alpha)-vWF tether bond. Biophys. J. 83, 194–205 (2002).
Whittaker, C.A. & Hynes, R.O. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell 13, 3369–3387 (2002).
Sakariassen, K.S., Bolhuis, P.A. & Sixma, J.J. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII–Von Willebrand factor bound to the subendothelium. Nature 279, 636–638 (1979).
Canobbio, I., Balduini, C. & Torti, M. Signalling through the platelet glycoprotein Ib-V-IX complex. Cell. Signal. 16, 1329–1344 (2004).
Offermanns, S. Activation of platelet function through G protein-coupled receptors. Circ. Res. 99, 1293–1304 (2006).
Nieswandt, B. & Watson, S.P. Platelet-collagen interaction: is GPVI the central receptor? Blood 102, 449–461 (2003).
Shattil, S.J. & Newman, P.J. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood 104, 1606–1615 (2004).
Hodivala-Dilke, K.M. et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 103, 229–238 (1999).
Lopez, A.D., Mathers, C.D., Ezzati, M., Jamison, D.T. & Murray, C.J. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367, 1747–1757 (2006).
Hankey, G.J. & Eikelboom, J.W. Antiplatelet drugs. Med. J. Aust. 178, 568–574 (2003).
Bennett, J.S. Novel platelet inhibitors. Annu. Rev. Med. 52, 161–184 (2001).
Coughlin, S.R. Thrombin signalling and protease-activated receptors. Nature 407, 258–264 (2000).
Ruggeri, Z.M., Orje, J.N., Habermann, R., Federici, A.B. & Reininger, A.J. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 108, 1903–1910 (2006).
Fukuda, K., Doggett, T., Laurenzi, I.J., Liddington, R.C. & Diacovo, T.G. The snake venom protein botrocetin acts as a biological brace to promote dysfunctional platelet aggregation. Nat. Struct. Mol. Biol. 12, 152–159 (2005).
Dumas, J.J. et al. Crystal structure of the wild-type von Willebrand factor A1-glycoprotein Ibalpha complex reveals conformation differences with a complex bearing von Willebrand disease mutations. J. Biol. Chem. 279, 23327–23334 (2004).
Huizinga, E.G. et al. Structures of glycoprotein Ibalpha and its complex with von Willebrand factor A1 domain. Science 297, 1176–1179 (2002).
Bogan, A.A. & Thorn, K.S. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 280, 1–9 (1998).
Shen, Y. et al. Requirement of leucine-rich repeats of glycoprotein (GP) Ibalpha for shear-dependent and static binding of von Willebrand factor to the platelet membrane GP Ib-IX-V complex. Blood 95, 903–910 (2000).
Fredrickson, B.J., Dong, J.F., McIntire, L.V. & Lopez, J.A. Shear-dependent rolling on von Willebrand factor of mammalian cells expressing the platelet glycoprotein Ib-IX-V complex. Blood 92, 3684–3693 (1998).
Furie, B. & Furie, B.C. Thrombus formation in vivo. J. Clin. Invest. 115, 3355–3362 (2005).
Ware, J., Russell, S. & Ruggeri, Z.M. Generation and rescue of a murine model of platelet dysfunction: the Bernard-Soulier syndrome. Proc. Natl. Acad. Sci. USA 97, 2803–2808 (2000).
Bergmeier, W. et al. The role of platelet adhesion receptor GPIbalpha far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc. Natl. Acad. Sci. USA 103, 16900–16905 (2006).
Denis, C. et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc. Natl. Acad. Sci. USA 95, 9524–9529 (1998).
Diacovo, T.G., Puri, K.D., Warnock, R.A., Springer, T.A. & von Andrian, U.H. Platelet-mediated lymphocyte delivery to high endothelial venules. Science 273, 252–255 (1996).
Collaborative Computational Project. No. 4 The CCP4 suite: programs for protein crystallography. Acta Crystallogr.D 50, 760–763 (1994).
Esnouf, R.M. An extensively modified version of Molscript that includes greatly enhanced coloring capabilities. J. Mol. Graph. Model. 15, 132–136 (1997).
Acknowledgements
We thank David Andrews (University of Miami) for performing VWF multimer gel analysis and Jeffrey S. Jhang (Special Hematology and Coagulation Laboratory, Columbia University Medical Center) for performing factor VIII function analysis. We thank B. Coller (Rockefeller University, New York) for providing the function-blocking mAbs 6D1 and 7E3, and R. Montgomery (Blood Center of Wisconsin, Milwaukee) for the mAb AP-1. This research is supported by National Institutes of Health grants HL063244, HL073646, and CA119342.
Author information
Authors and Affiliations
Contributions
J.C. generated the mutant VWF mice and performed experiments; K.T. performed Southern blot analysis and genotyping of mice; H.Z. bred, screened, maintained the animal colony and performed experiments; H.-F.L. sequenced genomic DNA, maintained the animal colony and performed experiments; D.T.-L.R. provided αIIb−/− animals; R.C.L. generated atomic models and contributed to the writing of the manuscript; T.G.D. cloned the VWF gene, performed experiments and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 and Figures 1–3 (PDF 2259 kb)
Supplementary Video 1
Laser-induced thrombus formation in arterioles contained within the cremaster muscle of a VWFR1326H mutant mouse. (MOV 2435 kb)
The ability of human platelets to preferentially support thrombus formation in the VWFR1326H mutant mouse was monitored in vivo by using 2-channel confocal intravital microscopy. Purified human platelets were labeled with BCECF ex-vivo, and mouse platelets with rhodamine 6G by intravenous administration. Upon induction of laser damage to the arterial vessel wall (A), human (green), and not mouse platelets (red), rapidly adhered to the site of injury, forming a large thrombus composed mainly of human cells. Rhodamine-labeled white blood cells can be seen interacting with the vessel wall in a post-capillary venule (V) next to the arterial.
Supplementary Video 2
Laser-induced thrombus formation in arterioles contained within the cremaster muscle of a WT mouse. (MOV 2963 kb)
Simultaneous monitoring of human (green) and mouse (red) platelet behavior in an animal possessing WT VWF reveals the lack of ability of human platelets to significantly participate in thrombus formation.
Supplementary Video 3
Human platelets can form occlusive arterial thrombi in VWF1326R>H mice despite the absence of αIIbβ3 on murine platelets. (MOV 2065 kb)
Mutant VWF-A1 mice were bred with αIIb null animals to generate the following genotype: Homozygous VWF1326R>H / αIIb -/-. Purified human platelets, labeled with BCECF ex-vivo, were infused continuously into the animal through a catheter inserted in the femoral artery. Vascular injury was induced by subjecting the arteriole to 5 second of continuous laser light (sweeping motion across the entire diameter of the vessel) at an intensity just below that required to rupture the vessel wall.
Rights and permissions
About this article
Cite this article
Chen, J., Tan, K., Zhou, H. et al. Modifying murine von Willebrand factor A1 domain for in vivo assessment of human platelet therapies. Nat Biotechnol 26, 114–119 (2008). https://doi.org/10.1038/nbt1373
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nbt1373
This article is cited by
-
Humanizing mouse thrombi
Nature Biotechnology (2008)