Abstract
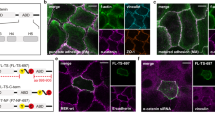
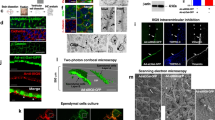
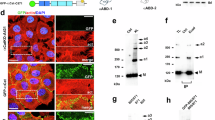
Epithelial cell junctions are essential for cell polarity, adhesion and morphogenesis. We have analysed VAB-9, a cell junction protein in Caenorhabditis elegans. VAB-9 is a predicted four-pass integral membrane protein that has greatest similarity to BCMP1 (brain cell membrane protein 1, a member of the PMP22/EMP/Claudin family of cell junction proteins) and localizes to the adherens junction domain of C. elegans apical junctions1,2,3,4. Here, we show that VAB-9 requires HMR-1/cadherin for localization to the cell membrane, and both HMP-1/α-catenin and HMP-2/β-catenin for maintaining its distribution at the cell junction. In vab-9 mutants, morphological defects correlate with disorganization of F-actin at the adherens junction; however, localization of the cadherin–catenin complex and epithelial polarity is normal. These results suggest that VAB-9 regulates interactions between the cytoskeleton and the adherens junction downstream of or parallel to α-catenin and/or β-catenin. Mutations in vab-9 enhance adhesion defects through functional loss of the cell junction genes apical junction molecule 1 (ajm-1) and discs large 1 (dlg-1), suggesting that VAB-9 is involved in cell adhesion. Thus, VAB-9 represents the first characterized tetraspan adherens junction protein in C. elegans and defines a new family of such proteins in higher eukaryotes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Notterpek, L. et al. Peripheral myelin protein 22 is a constituent of intercellular junctions in epithelia. Proc. Natl Acad. Sci. USA 98, 14404–14409 (2001).
Taylor, V., Welcher, A.A., Program, A.E. & Suter, U. Epithelial membrane protein-1, peripheral myelin protein 22, and lens membrane protein 20 define a novel gene family. J. Biol. Chem. 270, 28824–28833 (1995).
Christophe-Hobertus, C., Szpirer, C., Guyon, R. & Christophe, D. Identification of the gene encoding Brain Cell Membrane Protein I (BCMPI), a putative four-transmembrane protein distantly related to the Peripheral Myelin Protein 22/Epithelial Membrane Proteins and the Claudins. BMC Genomics 2, 3 (2001).
Tsukita, S., Furuse, M. & Itoh, M. Multifunctional strands in tight junctions. Nature Rev. Mol. Cell Biol. 2, 285–293 (2001).
Farquhar, M.G. & Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412 (1963).
Yeaman, C., Grindstaff, K., Hansen, M. & Nelson, W. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 79, 73–98 (1999).
Costa, M. et al. A putative catenin–cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J. Cell Biol. 141, 297–308 (1998).
Raich, W.B., Agbunag, C. & Hardin, J. Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr. Biol. 9, 1139–1146 (1999).
McMahon, L., Legouis, R., Vonesch, J.L. & Labouesse, M. Assembly of C. elegans apical junctions involves positioning and compaction by LET-413 and protein aggregation by the MAGUK protein DLG-1. J. Cell Sci. 114, 2265–2277 (2001).
Legouis, R. et al. LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nature Cell Biol. 2, 415–422 (2000).
Bossinger, O., Klebes, A., Segbert, C., Theres, C. & Knust, E. Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev. Biol. 230, 29–42 (2001).
Firestein, B.L. & Rongo, C. DLG-1 is a MAGUK similar to SAP97 and is required for adherens junction formation. Mol. Biol. Cell 12, 3465–3475 (2001).
Koppen, M. et al. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nature Cell Biol. 3, 983–991 (2001).
Knust, E. & Bossinger, O. Composition and formation of intercellular junctions in epithelial cells. Science 298, 1955–1959 (2002).
Baumgartner, S. et al. A Drosophila neurexin is required for septate junction and blood–nerve barrier formation and function. Cell 87, 1059–1068 (1996).
Lamb, R.S., Ward, R.E., Schweizer, L. & Fehon, R.G. Drosophila coracle, a member of the protein 4.1 superfamily, has essential functions in the septate junction and developmental functions in embryonic and adult epithelial cells. Mol. Biol. Cell 9, 3505–3519 (1998).
Mohler, W.A., Simske, J.S., Williams-Masson, E.M., Hardin, J.D. & White, J.G. Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr. Biol. 8, 1087–1090 (1998).
Priess, J.R. & Hirsh, D.I. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev. Biol. 117, 156–173 (1986).
Furuse, M., Fujita, K., Hiiragi, T., Fujimoto, K. & Tsukita, S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 141, 1539–1550 (1998).
Furuse, M., Sasaki, H., Fujimoto, K. & Tsukita, S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 143, 391–401 (1998).
Hresko, M.C., Williams, B.D. & Waterston, R.H. Assembly of body wall muscle and muscle cell attachment structures in Caenorhabditis elegans. J. Cell Biol. 124, 491–506 (1994).
Karabinos, A., Schmidt, H., Harborth, J., Schnabel, R. & Weber, K. Essential roles for four cytoplasmic intermediate filament proteins in Caenorhabditis elegans development. Proc. Natl Acad. Sci. USA 98, 7863–7868 (2001).
Herman, R. & Horvitz, H. in Nematodes as Biological Models, Volume 1: Behavioral and Developmental Models. (ed. Zuckerman, B.) 227–262 (Academic Press, New York, 1980).
Mohler, W.A. & White, J.G. Stereo-4-D reconstruction and animation from living fluorescent specimens. Biotechniques 24, 1006–1010, 1012 (1998).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Hodgkin, J. Male phenotypes and mating efficiency in C. elegans. Genetics 103, 43–64 (1983).
Williams, B.D., Schrank, B., Huynh, C., Shownkeen, R. & Waterston, R.H. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics 131, 609–624 (1992).
Stringham, E.G., Dixon, D.K., Jones, D. & Candido, E.P. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol. Biol. Cell 3, 221–233 (1992).
Wang, X. et al. Multiple ephrins control cell organization in C. elegans using kinase-dependent and -independent functions of the VAB-1 Eph receptor. Mol. Cell 4, 903–913 (1999).
Williams-Masson, E.M., Malik, A.N. & Hardin, J. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development 124, 2889–2901 (1997).
Moorthy, S., Chen, L. & Bennett, V. Caenorhabditis elegans β-G spectrin is dispensable for establishment of epithelial polarity, but essential for muscular and neuronal function. J. Cell Biol. 149, 915–930 (2000).
Bilder, D. & Perrimon, N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403, 676–680 (2000).
Acknowledgements
The authors wish to thank J. Priess and members of the Priess lab for providing lab space, research materials and intellectual input. We specifically thank J. Priess for suggesting the use of tetraploid strain SP346 to obtain larger embryos. We thank members of the Hardin lab, M. Labouesse, L. Bruggeman, D. Sonneborn and Joe for helpful discussions. We thank J. White and W. Mohler for assistance with MPLSM, D. Hall for advice with TEM, A. Chisholm for the vab-9 (ju6) strain, P. Heid for cosmid pools, and A. Fire for GFP and heat-shock vectors. Some of the nematode strains used in this study were provided by the Caenorhabditis Genetics Centre, which is funded by the NIH National Centre for Research Resources (NCRR). The Nematode Expression Database (http://nematode.lab.nig.ac.jp/) and some cDNA clones were provided courtesy of the laboratory of Y. Kohara at the National Institute of Genetics, Japan. This work was supported by NIH grant GM58038 (J.D.H.). J.S.S. was a Helen Hay Whitney fellow. Some funds were provided by the Gamete and Embryo Training Grant from the University of Wisconsin. The Bio-Rad MRC 1024 confocal microscope at the University of Wisconsin is supported by National Science Foundation grant 9724515.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Simske, J., Köppen, M., Sims, P. et al. The cell junction protein VAB-9 regulates adhesion and epidermal morphology in C. elegans. Nat Cell Biol 5, 619–625 (2003). https://doi.org/10.1038/ncb1002
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ncb1002
This article is cited by
-
Occluding junctions of invertebrate epithelia
Journal of Comparative Physiology B (2016)
-
Targeted knockout and lacZ reporter expression of the mouse Tmhs deafness gene and characterization of the hscy-2J mutation
Mammalian Genome (2007)
-
TM4SF10 gene sequencing in XLMR patients identifies common polymorphisms but no disease-associated mutation
BMC Medical Genetics (2004)
-
α-catenin: at the junction of intercellular adhesion and actin dynamics
Nature Reviews Molecular Cell Biology (2004)