Abstract
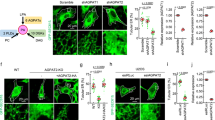
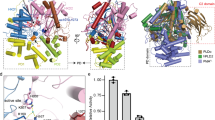
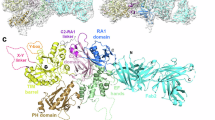
Dynamin is a large GTP-binding protein that mediates endocytosis by hydrolyzing GTP1,2,3. Previously, we reported that phospholipase D2 (PLD2) interacts with dynamin in a GTP-dependent manner4. This implies that PLD may regulate the GTPase cycle of dynamin. Here, we show that PLD functions as a GTPase activating protein (GAP) through its phox homology domain (PX), which directly activates the GTPase domain of dynamin, and that the arginine residues in the PLD–PX are vital for this GAP function. Moreover, wild-type PLD–PX, but not mutated PLD–PXs defective for GAP function in vitro, increased epidermal growth factor receptor (EGFR) endocytosis at physiological EGF concentrations. In addition, the silencing of PLDs was shown to retard EGFR endocytosis and the addition of wild-type PLDs or lipase-inactive PLDs, but not PLD1 mutants with defective GAP activity for dynamin in vitro, resulted in the recovery of EGFR endocytosis. These findings suggest that PLD, functioning as an intermolecular GAP for dynamin, accelerates EGFR endocytosis. Moreover, we determined that the phox homology domain itself had GAP activity — a novel function in addition to its role as a binding motif for proteins or lipids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schmid, S. L., McNiven, M. A. & De Camilli, P. Dynamin and its partners: a progress report. Curr. Opin. Cell Biol. 10, 504–512 (1998).
Henley, J. R., Krueger, E. W., Oswald, B. J. & McNiven, M. A. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141, 85–99 (1998).
Hinshaw, J. E. Dynamin and its role in membrane fission. Annu. Rev. Cell Dev. Biol. 16, 483–519 (2000).
Park, J. B. et al. H. Regulation of phospholipase D2 by GTP-dependent interaction with dynamin. Adv. Enzyme Regul. 44, 249–264 (2004).
Hammond, S. M. et al. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J. Biol. Chem. 270, 29640–29643 (1995).
Colley, W. C. et al. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 7, 191–201 (1997).
Frohman, M. A., Sung, T. C. & Morris, A. J. Mammalian phospholipase D structure and regulation. Biochim. Biophys. Acta. 1439, 175–186 (1999).
Exton, J. H. Regulation of phospholipase D. FEBS Lett. 531, 58–61 (2002).
Park, J. B. et al. Cardiac phospholipase D2 localizes to sarcolemmal membranes and is inhibited by α-actinin in an ADP-ribosylation factor-reversible manner. J. Biol. Chem. 275, 21295–21301 (2000).
Jang, I. H. et al. The direct interaction of phospholipase C-γ1 with phospholipase D2 is important for epidermal growth factor signaling. J. Biol. Chem. 278, 18184–18190 (2003).
Warnock, D. E. & Schmid, S. L. Dynamin GTPase, a force-generating molecular switch. Bioessays 18, 885–893 (1996).
Scheffzek, K., Ahmadian, M. R. & Wittinghofer, A. GTPase-activating proteins: helping hands to complement an active site. Trends Biochem. Sci. 23, 257–262 (1998).
Sever, S., Muhlberg, A B., & Schmid, S. L. Impairment of dynamin's GAP domain stimulates receptor-mediated endocytosis. Nature 398, 481–486 (1999).
Ellson, C. D., Andrews, S., Stephens, L. R. & Hawkins, P. T. The PX domain: a new phosphoinositide-binding module. J. Cell Sci. 115, 1099–1105 (2002).
Karathanassis, D. et al. Binding of the PX domain of p47(phox) to phosphatidylinositol 3,4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J. 21, 5057–5068 (2002).
Tuma, P. L., Stachniak, M. C. & Collins, C. A. Activation of dynamin GTPase by acidic phospholipids and endogenous rat brain vesicles. J. Biol. Chem. 268, 17240–17246 (1993).
Barylko, B. et al. Synergistic activation of dynamin GTPase by Grb2 and phosphoinositides. J. Biol. Chem. 273, 3791–3797 (1998).
Song, B. D. & Schmid, S. L. A molecular motor or a regulator? Dynamin's in a class of its own. Biochemistry 42, 1369–1376 (2003).
Marks, B. et al. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature 410, 231–235 (2001).
Du, G., Huang, P., Liang, B. T. & Frohman, M. A. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol. Biol. Cell. 15, 1024–1030 (2004).
Shen, Y., Xu, L. & Foster, D. A. Role for phospholipase D in receptor-mediated endocytosis. Mol. Cell. Biol. 21, 595–602 (2001).
Lembach, K. J. Induction of human fibroblast proliferation by epidermal growth factor (EGF): enhancement by an EGF-binding arginine esterase and by ascorbate. Proc. Natl Acad. Sci. USA 73, 183–187 (1976).
Li, G. et al. Insulin at physiological concentrations selectively activates insulin but not insulin like growth factor (IGF-1) or insulin/IGF-1 hybrid receptors in endothelial cells. Endocrinology 146, 4690–4696 (2005).
Freyberg, Z., Siddhanta, A. & Shields, D. “Slip, sliding away”: phospholipase D and the Golgi apparatus. Trends Cell Biol. 13, 540–546 (2003).
Cockcroft, S. Signalling roles of mammalian phospholipase D1 and D2. Cell. Mol. Life. Sci. 58, 1674–1687 (2001).
Damke, H., Baba, T., Warnock, D. E. & Schmid S. L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127, 915–934 (1994).
Kim, J. H., Kim, J. H., Ohba, M., Suh, P. G. & Ryu, S. H. Novel functions of the phospholipase D2-Phox homology domain in protein kinase Cζ activation. Mol. Cell. Biol. 25, 3194–3208 (2005).
Robinson, P., J. et al. Dynamin GTPase regulated by protein kinase C phosphorylation in nerve terminal. Nature 365, 163–166 (1993).
Kim, Y., et al. Phosphorylation and activation of phospholipase D1 by protein kinase C in vivo: determination of multiple phosphorylation sites. Biochemistry 38, 10344–1035 (1999).
Vieira, A. V., Lamaze, C. & Schmid, S. L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274, 2086–2089 (1996).
Acknowledgements
This work was supported in part by a grant from the 21st Frontier Functional Proteomics Research (M102KM010001–03K1301–00710) and by the Ministry of Science and Technology (MOST) through the Systems Bio-Dynamics Research Center in the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3 and S4 (PDF 522 kb)
Rights and permissions
About this article
Cite this article
Lee, C., Kim, I., Park, J. et al. The phox homology domain of phospholipase D activates dynamin GTPase activity and accelerates EGFR endocytosis. Nat Cell Biol 8, 477–484 (2006). https://doi.org/10.1038/ncb1401
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ncb1401
This article is cited by
-
Phospholipase D2 is a positive regulator of sirtuin 1 and modulates p53-mediated apoptosis via sirtuin 1
Experimental & Molecular Medicine (2021)
-
Physiological function of phospholipase D2 in anti-tumor immunity: regulation of CD8+ T lymphocyte proliferation
Scientific Reports (2018)
-
Phospholipase D2 loss results in increased blood pressure via inhibition of the endothelial nitric oxide synthase pathway
Scientific Reports (2017)
-
IRAS Modulates Opioid Tolerance and Dependence by Regulating μ Opioid Receptor Trafficking
Molecular Neurobiology (2016)
-
The pleckstrin homology domain of phospholipase D1 accelerates EGFR endocytosis by increasing the expression of the Rab5 effector, rabaptin-5
Experimental & Molecular Medicine (2015)