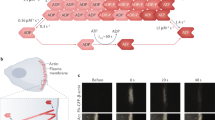
Abstract
Physical force evokes rearrangement of the actin cytoskeleton. Signalling pathways such as tyrosine kinases, stretch-activated Ca2+ channels and Rho GTPases are involved in force sensing. However, how signals are transduced to actin assembly remains obscure. Here we show mechanosensitive actin polymerization by formins (formin homology proteins). Cells overexpressing mDia1 increased the amount of F-actin on release of cell tension. Fluorescence single-molecule speckle microscopy revealed rapid induction of processive actin assembly by mDia1 on cell cortex deformation. mDia1 lacking the Rho-binding domain and other formins exhibited mechanosensitive actin nucleation, suggesting Rho-independent activation. Mechanosensitive actin nucleation by mDia1 required neither Ca2+ nor kinase signalling. Overexpressing LIM kinase abrogated the induction of processive mDia1. Furthermore, s-FDAPplus (sequential fluorescence decay after photoactivation) analysis revealed a rapid actin monomer increase on cell cortex deformation. Our direct visualization of the molecular behaviour reveals a mechanosensitive actin filament regeneration mechanism in which G-actin released by actin remodelling plays a pivotal role.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Birukov, K. G. Small GTPases in mechanosensitive regulation of endothelial barrier. Microvasc. Res. 77, 46–52 (2009).
Tzima, E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ. Res. 98, 176–185 (2006).
Shirinsky, V. P. et al. Mechano-chemical control of human endothelium orientation and size. J. Cell Biol. 109, 331–339 (1989).
Davies, P. F., Robotewskyj, A. & Griem, M. L. Quantitative studies of endothelial cell adhesion. Directional remodelling of focal adhesion sites in response to flow forces. J. Clin. Invest. 93, 2031–2038 (1994).
Martin, P. & Lewis, J. Actin cables and epidermal movement in embryonic wound healing. Nature 360, 179–183 (1992).
Vogel, V. & Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7, 265–275 (2006).
Hoffman, B. D., Grashoff, C. & Schwartz, M. A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323 (2011).
Wojciak-Stothard, B. & Ridley, A. J. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J. Cell Biol. 161, 429–439 (2003).
Kaunas, R., Nguyen, P., Usami, S. & Chien, S. Cooperative effects of Rho and mechanical stretch on stress fibre organization. Proc. Natl Acad. Sci. USA 102, 15895–15900 (2005).
Li, S. et al. Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J. Clin. Invest. 103, 1141–1150 (1999).
Li, F. & Higgs, H. N. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 13, 1335–1340 (2003).
Rose, R. et al. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature 435, 513–518 (2005).
Watanabe, N., Kato, T., Fujita, A., Ishizaki, T. & Narumiya, S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1, 136–143 (1999).
Maiti, S. et al. Structure and activity of full-length formin mDia1. Cytoskeleton (Hoboken) 69, 393–405 (2012).
Romero, S. et al. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 119, 419–429 (2004).
Kovar, D. R., Harris, E. S., Mahaffy, R., Higgs, H. N. & Pollard, T. D. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell 124, 423–435 (2006).
Higashida, C. et al. Actin polymerization-driven molecular movement of mDia1 in living cells. Science 303, 2007–2010 (2004).
Watanabe, N. & Mitchison, T. J. Single-molecule speckle analysis of actin filament turnover in lamellipodia. Science 295, 1083–1086 (2002).
Watanabe, N. Inside view of cell locomotion through single-molecule: fast F-/G-actin cycle and G-actin regulation of polymer restoration. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 86, 62–83 (2010).
Watanabe, N. Fluorescence single-molecule imaging of actin turnover and regulatory mechanisms. Methods Enzymol. 505, 219–232 (2012).
Higashida, C. et al. G-actin regulates rapid induction of actin nucleation by mDia1 to restore cellular actin polymers. J. Cell Sci. 121, 3403–3412 (2008).
Kiuchi, T., Nagai, T., Ohashi, K. & Mizuno, K. Measurements of spatiotemporal changes in G-actin concentration reveal its effect on stimulus-induced actin assembly and lamellipodium extension. J. Cell Biol. 193, 365–380 (2011).
Miyoshi, T. et al. Actin turnover-dependent fast dissociation of capping protein in the dendritic nucleation actin network: evidence of frequent filament severing. J. Cell Biol. 175, 947–955 (2006).
Munevar, S., Wang, Y. L. & Dembo, M. Regulation of mechanical interactions between fibroblasts and the substratum by stretch-activated Ca2+ entry. J. Cell Sci. 117, 85–92 (2004).
Yang, X. C. & Sachs, F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science 243, 1068–1071 (1989).
Lee, J., Ishihara, A., Oxford, G., Johnson, B. & Jacobson, K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature 400, 382–386 (1999).
Jackson, T. R., Patterson, S. I., Thastrup, O. & Hanley, M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem. J. 253, 81–86 (1988).
Giannone, G. & Sheetz, M. P. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 16, 213–223 (2006).
Sawada, Y. et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026 (2006).
Chaturvedi, L. S., Marsh, H. M., Shang, X., Zheng, Y. & Basson, M. D. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J. Biol. Chem. 282, 14–28 (2007).
Fabian, M. A. et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 23, 329–336 (2005).
Nakano, H. et al. Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60. J. Antibiot. (Tokyo) 40, 706–708 (1987).
Hennequin, L. F. et al. N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5-(tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J. Med. Chem. 49, 6465–6488 (2006).
Li, J. et al. A chemical and phosphoproteomic characterization of dasatinib action in lung cancer. Nat. Chem. Biol. 6, 291–299 (2010).
Slack-Davis, J. K. et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J. Biol. Chem. 282, 14845–14852 (2007).
Brandvold, K. R., Steffey, M. E., Fox, C. C. & Soellner, M. B. Development of a Highly Selective c-Src Kinase Inhibitor. ACS Chem. Biol. 7, 1393–1398 (2012).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 (2008).
Tsuji, T., Miyoshi, T., Higashida, C., Narumiya, S. & Watanabe, N. An order of magnitude faster AIP1-associated actin disruption than nucleation by the Arp2/3 complex in lamellipodia. PLoS ONE 4, e4921 (2009).
Okada, K. et al. Xenopus actin-interacting protein 1 (XAip1) enhances cofilin fragmentation of filaments by capping filament ends. J. Biol. Chem. 277, 43011–43016 (2002).
Kiuchi, T., Ohashi, K., Kurita, S. & Mizuno, K. Cofilin promotes stimulus-induced lamellipodium formation by generating an abundant supply of actin monomers. J. Cell Biol. 177, 465–476 (2007).
Kiuchi, T., Nagai, T., Ohashi, K., Watanabe, N. & Mizuno, K. Live-cell imaging of G-actin dynamics using sequential FDAP. Bioarchitecture 1, 240–244 (2011).
Ando, R., Mizuno, H. & Miyawaki, A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science 306, 1370–1373 (2004).
Yang, N. et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 393, 809–812 (1998).
Hotulainen, P., Paunola, E., Vartiainen, M. K. & Lappalainen, P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol. Biol. Cell 16, 649–664 (2005).
Nishida, M. et al. P2Y6 receptor-G α12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 27, 3104–3115 (2008).
Krepinsky, J. C. et al. Nitric oxide inhibits stretch-induced MAPK activation in mesangial cells through RhoA inactivation. J. Am. Soc. Nephrol. 14, 2790–2800 (2003).
Pan, J. et al. PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J. Cell Physiol. 202, 536–553 (2005).
Ko, K. S., Arora, P. D. & McCulloch, C. A. Cadherins mediate intercellular mechanical signalling in fibroblasts by activation of stretch-sensitive calcium-permeable channels. J. Biol. Chem. 276, 35967–35977 (2001).
Chen, X. Z. et al. Submembraneous microtubule cytoskeleton: interaction of TRPP2 with the cell cytoskeleton. FEBS J. 275, 4675–4683 (2008).
Sharif-Naeini, R. et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell 139, 587–596 (2009).
Guan, J. L. Role of focal adhesion kinase in integrin signalling. Int. J. Biochem. Cell Biol. 29, 1085–1096 (1997).
Sadoshima, J. & Izumo, S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 59, 551–571 (1997).
Hayakawa, K., Tatsumi, H. & Sokabe, M. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J. Cell Biol. 195, 721–727 (2011).
McCullough, B.R. et al. Cofilin-linked changes in actin filament flexibility promote severing. Biophys. J. 101, 151–9 (2011).
Mizuno, H. et al. Rotational movement of the formin mDia1 along the double helical strand of an actin filament. Science 331, 80–83 (2011).
Mizuno, H. & Watanabe, N. mDia1 and formins: screw cap of the actin filament. Biophysics 8, 95–102 (2012).
Yarmola, E. G., Somasundaram, T., Boring, T. A., Spector, I. & Bubb, M. R. Actin-latrunculin A structure and function. Differential modulation of actin-binding protein function by latrunculin A. J. Biol. Chem. 275, 28120–28127 (2000).
Weber, A., Nachmias, V. T., Pennise, C. R., Pring, M. & Safer, D. Interaction of thymosin beta 4 with muscle and platelet actin: implications for actin sequestration in resting platelets. Biochemistry 31, 6179–6185 (1992).
Yarmola, E. G., Parikh, S. & Bubb, M. R. Formation and implications of a ternary complex of profilin, thymosin beta 4, and actin. J. Biol. Chem. 276, 45555–45563 (2001).
Smith, M. B. et al. Interactive, computer-assisted tracking of speckle trajectories in fluorescence microscopy: application to actin polymerization and membrane fusion. Biophys. J. 101, 1794–1804 (2011).
Stiel, A. C. et al. 1.8 A bright-state structure of the reversibly switchable fluorescent protein Dronpa guides the generation of fast switching variants. Biochem. J. 402, 35–42 (2007).
Chang, H. et al. A unique series of reversibly switchable fluorescent proteins with beneficial properties for various applications. Proc. Natl Acad. Sci. USA 109, 4455–4460 (2012).
Acknowledgements
We thank M. B. Smith and D. Vavylonis for development of Speckle TrackerJ. This work was supported by the Cabinet Office, Government of Japan through the Funding Program for Next Generation World-Leading Researchers (LS013) and by grants from the Human Frontier Science Program and the Takeda Science Foundation. C.H. was a JSPS research fellow.
Author information
Authors and Affiliations
Contributions
C.H., T.K. and N.W. planned the experimental design and wrote the paper. C.H., T.K., Y.A., H.M., M.M. and N.W. conducted the experiments and analysed the data. S.N. and K.M. contributed reagents and supervised this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1391 kb)
Supplementary Table 1
Supplementary Information (XLSX 26 kb)
Supplementary Table 2
Supplementary Information (XLSX 30 kb)
Application of mechanical stress by manipulating microneedle.
A microneedle was placed on top of the nuclear region and was laterally moved for several seconds before acquiring fluorescence images (Fig. 2a). Time is in minute:second. Scale bar, 20 μm. (MOV 3183 kb)
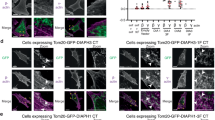
Needle manipulation induced processive movement of EGFP-mDia1Full.
The left and right movies show time-lapse images of mDia1Full speckles taken before (top) and 9 s after treatment (bottom) (Fig. 2b). The left and right movies are identical except that in the left movies, red circles indicate the speckles moving in one direction for at least five consecutive frames. Only a few mDia1Full speckles exhibited processive movement before needle manipulation. The density of mDia1Full speckles moving processively increased after manipulation. Time is in second. (MOV 1074 kb)
Needle manipulation induced an increase in the number of processively moving speckles of EGFP-XDia1.
The left and right movies show images before and 9 s after manipulation, respectively (Fig. 2d). Red circles indicate the speckles moving in one direction for at least five consecutive frames. Time is in second. Scale bar, 5 μm. (MOV 453 kb)
Needle manipulation induced an increase in the number of processively moving speckles of EGFP-XINF2.
The left and right movies show images before and 10 s after needle manipulation, respectively (Fig. 3a). Red circles indicate the speckles moving in one direction for at least five consecutive frames. Time is in second. Scale bar, 5 μm. (MOV 457 kb)
The number of processively moving speckles of EGFP-XFRL1 (formin-related gene in leukocytes) increased after needle manipulation.
The left and right movies show images before and 9 s after needle manipulation, respectively (Fig. 3b). Red circles show the speckles moving in one direction for at least five consecutive frames. Time is in second. Scale bar, 5 μm. (MOV 469 kb)
Needle manipulation increased a frequency of processively moving EGFP-mDia1ΔN3.
The left and right movies show time-lapse images of mDia1ΔN3 speckles taken before (top) and 7 s after (bottom) needle manipulation (Fig. 3c). The density of mDia1ΔN3 speckles moving processively increased after manipulation. Red dots in the left movies indicate the speckles moving in one direction for at least five consecutive frames. Time is in second. Scale bar, 2 μm. (MOV 403 kb)
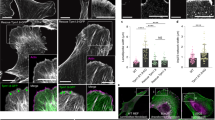
Needle manipulation increased a frequency of processively moving EGFP-mDia1 FH2.
The left and right movies show time-lapse images of mDia1-FH2 (a.a. 736-1192) speckles taken before and 10 s after needle manipulation (Fig. 3d). Red circles (50 on the left; 102 on the right) indicate the speckles moving in one direction for at least five consecutive frames. The density of mDia1-FH2 speckles moving processively increased after manipulation. Time is in second. Scale bar, 5 μm. (MOV 1842 kb)
The number of EGFP-RhoA speckles increased after needle manipulation.
The left and right movies show time-lapse images of EGFP-RhoA speckles taken before and 9 s after needle manipulation, respectively (Fig. 3e). The density of EGFP-RhoA speckles increased after needle manipulation. RhoA speckles moving in one direction at least for five consecutive frames are indicated by red circles. Time is in second. (MOV 561 kb)
mDia1Full processive speckles did not increase upon 500 nM A23187 treatment.
The left and right movies show time-lapse images of mDia1Full speckles taken before and 5 min after 500 nM A23187 treatment, respectively (Fig. 4a, top panels). Induction of processively speckles was not observed by A23187 treatment. Time is in second. Scale bar, 2 μm. (MOV 729 kb)
mDia1ΔN3 processive speckles did not increase upon 500 nM A23187 treatment.
The upper and lower movies show time-lapse images of mDia1ΔN3 speckles taken before and 5 min after 500 nM A23187 treatment, respectively (Fig. 4a, bottom panels). Red dots in the left movies show the speckles moving in one direction for at least five consecutive frames. Time is in second. Scale bar, 2 μm. (MOV 592 kb)
Induction of proccesively-moving speckles of mDia1Full by needle manipulation was not inhibited by EGTA.
The upper and lower movies show time-lapse images of mDia1Full speckles taken before and 12 s after needle manipulation (Fig. 4b). Cells were pretreated with 3 mM EGTA for 47 min. Red circles in the upper movies show the speckles moving in one direction for at least five consecutive frames (none was observed before manipulation). Time is in second. Scale bar, 2 μm. (MOV 779 kb)
Induction of proccesively-moving speckles of mDia1Full by needle manipulation was not inhibited by long-term thapsigargin treatment.
The upper and lower movies show time-lapse images of mDia1Full speckles taken before and 10 s after needle manipulation, respectively (Fig. 4c). Cells were pretreated with 2 μM thapsigargin for 60 min. Red circles in the left movies show the speckles moving in one direction for at least five consecutive frames. Time is in second. Scale bar, 2 μm. (MOV 2389 kb)
Thapsigargin did not inhibit induction of processive XDia1 speckles by needle manipulation.
The movies show time-lapse images of XDia1 speckles taken before (left) and 8 s after (right) needle manipulation (Fig. 4d). Cells were pretreated with 3 μM thapsigargin for 75 min before needle manipulation. Red circles show the speckles moving in one direction for at least five consecutive frames. Time is in second. Scale bar, 5 μm. (MOV 733 kb)
Processive speckles of mDia1Full increased after needle manipulation in the presence of 10 μM BAPTA-AM.
The left and right movies show time-lapse images of mDia1Full speckles taken before and 12 s after needle manipulation, respectively (another example of bottom data in Supplementary Fig. S1). Cells were pretreated with 10 μM BAPTA-AM for 55 min. Red circles show the speckles moving in one direction for at least five consecutive frames. Time is in second. (MOV 1232 kb)
Activation of mDia1Full was observed upon needle manipulation in the presence of 100 nM staurosporine.
The upper and lower movies show time-lapse images of mDia1Full speckles taken before and 6 s after needle manipulation, respectively (Fig. 5b, top). Cells were pretreated with 100 nM staurosporine for 41 min. Red dots in the left movies show the speckles moving in one direction for at least five consecutive frames. Time is in second. Scale bar, 5 μm. (MOV 2809 kb)
Activation mDia1ΔN3 was observed upon microneedle manipulation in the presence of 100 nM staurosporine.
The upper and lower movies show time-lapse images of mDia1Full speckles taken before and 10 s after needle manipulation, respectively (Fig. 5b, bottom). Cells were pretreated with 100 nM staurosporine for 42 min. Red dots in the left movies show the speckles moving in one direction for at least five consecutive frames. Time is in second. Scale bar, 2 μm. (MOV 1099 kb)
Induction of mDia1Full by needle manipulation in the presence of 10 μM AZD0530.
The left and right movies show time-lapse images of single-molecule mDia1Full speckles before and ≈10 s after needle manipulation, respectively (Fig. 5e, top left). Cells were pretreated with 1 μM AZD0530 for 3 h. Red circles indicate the speckles directionally moving for at least five consecutive frames. Scale bar, 5 μm. (MOV 1539 kb)
Induction of mDia1Full by needle manipulation in the presence of 10 nM dasatinib
The left and right movies show time-lapse images of single-molecule mDia1Full speckles before and ≈10 s after needle manipulation, respectively (Fig. 5e, top right). Cells were pretreated with 10 nM dasatinib for 4 h. Red circles indicate the speckles directionally moving for at least five consecutive frames. Scale bar, 5 μm. (MOV 984 kb)
Induction of mDia1Full by needle manipulation in the presence of 100 nM PF-228.
The left and right movies show time-lapse images of single-molecule mDia1Full speckles before and ≈10 s after needle manipulation, respectively (Fig. 5e, bottom left). Cells were pretreated with an FAK inhibitor PF-228 at 100 nM for 4 h. Red circles indicate the speckles directionally moving for at least five consecutive frames. Scale bar, 5 μm. (MOV 1689 kb)
Induction of mDia1Full by needle manipulation in the presence of 1 μM PP2.
The left and right movies show time-lapse images of single-molecule mDia1Full speckles before and ≈10 s after needle manipulation, respectively (Fig. 5e, bottom right). Cells were pretreated with 1 μM PP2 for 6 h. Red circles indicate the speckles directionally moving for at least five consecutive frames. Scale bar, 5 μm. (MOV 776 kb)
Rights and permissions
About this article
Cite this article
Higashida, C., Kiuchi, T., Akiba, Y. et al. F- and G-actin homeostasis regulates mechanosensitive actin nucleation by formins. Nat Cell Biol 15, 395–405 (2013). https://doi.org/10.1038/ncb2693
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ncb2693
This article is cited by
-
Emergence of periodic circumferential actin cables from the anisotropic fusion of actin nanoclusters during tubulogenesis
Nature Communications (2024)
-
Low-intensity pulsed ultrasound/nanomechanical force generators enhance osteogenesis of BMSCs through microfilaments and TRPM7
Journal of Nanobiotechnology (2022)
-
INF2-mediated actin filament reorganization confers intrinsic resilience to neuronal ischemic injury
Nature Communications (2022)
-
Optineurin links Hace1-dependent Rac ubiquitylation to integrin-mediated mechanotransduction to control bacterial invasion and cell division
Nature Communications (2022)
-
Colonization of distant organs by tumor cells generating circulating homotypic clusters adaptive to fluid shear stress
Scientific Reports (2021)