Abstract
Heterozygosity of the retinoblastoma gene Rb1 elicits tumorigenesis in susceptible tissues following spontaneous loss of the remaining functional allele. Inactivation of previously studied retinoblastoma protein (pRb) targets partially inhibited tumorigenesis in Rb1+/− mice1,2,3,4,5,6. Here we report that inactivation of pRb target Skp2 (refs. 7,8) completely prevents spontaneous tumorigenesis in Rb1+/− mice. Targeted Rb1 deletion in melanotrophs ablates the entire pituitary intermediate lobe when Skp2 is inactivated. Skp2 inactivation does not inhibit aberrant proliferation of Rb1-deleted melanotrophs but induces their apoptotic death. Eliminating p27 phosphorylation on T187 in p27T187A knock-in mice reproduces the effects of Skp2 knockout, identifying p27 ubiquitination by SCFSkp2 ubiquitin ligase as the underlying mechanism for Skp2's essential tumorigenic role in this setting. RB1-deficient human retinoblastoma cells also undergo apoptosis after Skp2 knockdown; and ectopic expression of p27, especially the p27T187A mutant, induces apoptosis. These results reveal that Skp2 becomes an essential survival gene when susceptible cells incur Rb1 deficiency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yamasaki, L. et al. Loss of E2F–1 reduces tumorigenesis and extends the lifespan of Rb1+/− mice. Nat. Genet. 18, 360–364 (1998).
Ziebold, U., Lee, E.Y., Bronson, R.T. & Lees, J.A. E2F3 loss has opposing effects on different pRB-deficient tumors, resulting in suppression of pituitary tumors but metastasis of medullary thyroid carcinomas. Mol. Cell. Biol. 23, 6542–6552 (2003).
Lee, E.Y. et al. E2F4 loss suppresses tumorigenesis in Rb mutant mice. Cancer Cell 2, 463–472 (2002).
Lasorella, A., Rothschild, G., Yokota, Y., Russell, R.G. & Iavarone, A. Id2 mediates tumor initiation, proliferation, and angiogenesis in Rb mutant mice. Mol. Cell. Biol. 25, 3563–3574 (2005).
Takahashi, C. et al. Nras loss induces metastatic conversion of Rb1-deficient neuroendocrine thyroid tumor. Nat. Genet. 38, 118–123 (2006).
Takahashi, C., Contreras, B., Bronson, R.T., Loda, M. & Ewen, M.E. Genetic interaction between Rb and K-ras in the control of differentiation and tumor suppression. Mol. Cell. Biol. 24, 10406–10415 (2004).
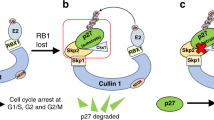
Ji, P. et al. An Rb-Skp2-p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol. Cell 16, 47–58 (2004).
Binne, U.K. et al. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat. Cell Biol. 9, 225–232 (2007).
Frescas, D. & Pagano, M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat. Rev. Cancer 8, 438–449 (2008).
Zhang, L. & Wang, C. F-box protein Skp2: a novel transcriptional target of E2F. Oncogene 25, 2615–2627 (2005).
Yung, Y., Walker, J.L., Roberts, J.M. & Assoian, R.K.A. Skp2 autoinduction loop and restriction point control. J. Cell Biol. 178, 741–747 (2007).
Jacks, T. et al. Effects of an Rb mutation in the mouse. Nature 359, 295–300 (1992).
Vooijs, M., van der Valk, M., te Riele, H. & Berns, A. Flp-mediated tissue-specific inactivation of the retinoblastoma tumor suppressor gene in the mouse. Oncogene 17, 1–12 (1998).
Nakayama, K. et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 19, 2069–2081 (2000).
Malek, N.P. et al. A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature 413, 323–327 (2001).
Carneiro, C. et al. p27 deficiency desensitizes Rb−/− cells to signals that trigger apoptosis during pituitary tumor development. Oncogene 22, 361–369 (2003).
Ji, P., Sun, D., Wang, H., Bauzon, F. & Zhu, L. Disrupting Skp2-cyclin A interaction with a blocking peptide induces selective cancer cell killing. Mol. Cancer Ther. 6, 684–691 (2007).
Kitagawa, M., Lee, S.H. & McCormick, F. Skp2 suppresses p53-dependent apoptosis by inhibiting p300. Mol. Cell 29, 217–231 (2008).
Xu, X.L. et al. Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specific MDM2 signaling. Cell 137, 1018–1031 (2009).
Leung, S.W. et al. A dynamic switch in Rb+/− mediated neuroendocrine tumorigenesis. Oncogene 23, 3296–3307 (2004).
Balthasar, N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–891 (2004).
Sage, J., Miller, A.L., Perez-Mancera, P.A., Wysocki, J.M. & Jacks, T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424, 223–228 (2003).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Timmerbeul, I. et al. Testing the importance of p27 degradation by the SCFskp2 pathway in murine models of lung and colon cancer. Proc. Natl. Acad. Sci. USA 103, 14009–14014 (2006).
Cobrinik, D., Francis, R.O., Abramson, D.H. & Lee, T.C. Rb induces a proliferative arrest and curtails Brn-2 expression in retinoblastoma cells. Mol. Cancer 5, 72 (2006).
Sheaff, R.J., Groudine, M., Gordon, M., Roberts, J.M. & Clurman, B.E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11, 1464–1478 (1997).
Acknowledgements
We thank J. Cui for expert technical assistance, R. Mahmood for help with tissue preparation and histological analysis, D. Abramson and S. Jhanwar for support of retinoblastoma cell analysis, A. Koff for comments on the manuscript and A. Burns and W. Zhang for encouragement. We are grateful to T. Jacks for providing Rb1+/− mice (from L. Yamasaki and A. Iavarone) and Rb1lox/lox mice, B. Lowell and S. Chua for POMC-Cre mice, J. Roberts for p27T187A knock-in mice and F. Costantini for Rosa26YFP mice (from J. Pollard). This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and National Cancer Institute of the US National Institutes of Health to L.Z. Albert Einstein Comprehensive Cancer Research Center and Liver Research Center provided core facility support. F.B. was supported by the Training Program in Cellular and Molecular Biology and Genetics (T32 GM007491) at the Albert Einstein College of Medicine. L.Z. is a recipient of the Irma T. Hirschl Career Scientist Award.
Author information
Authors and Affiliations
Contributions
H.W., P.J., F.B., D.S. and L.Z. designed and performed experiments with mice mutant for Rb1, Skp2, p27 or targeted deletion of Rb1. J.L. and R.S.S. performed pathology studies. D.C. and X.X. designed and performed analyses of retinoblastoma cells; H.W. performed protein blot experiments. K.N. and K.I.N. provided Skp2+/− mice. H.W., J.L., D.C. and L.Z. wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Table 1 (PDF 885 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Bauzon, F., Ji, P. et al. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/− mice. Nat Genet 42, 83–88 (2010). https://doi.org/10.1038/ng.498
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ng.498
This article is cited by
-
Akt: a key transducer in cancer
Journal of Biomedical Science (2022)
-
Targeting the untargetable: RB1-deficient tumours are vulnerable to Skp2 ubiquitin ligase inhibition
British Journal of Cancer (2022)
-
Functional genomics identifies new synergistic therapies for retinoblastoma
Oncogene (2020)
-
Modeling germline mutations in pineoblastoma uncovers lysosome disruption-based therapy
Nature Communications (2020)
-
Preclinical studies reveal MLN4924 is a promising new retinoblastoma therapy
Cell Death Discovery (2020)