Abstract
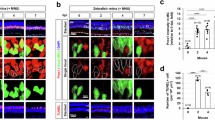
Although insights have emerged regarding genes controlling the early stages of eye formation, little is known about lens-fibre differentiation and elongation. The expression pattern of the Prox1 homeobox gene suggests it has a role in a variety of embryonic tissues, including lens1. To analyse the requirement for Prox1 during mammalian development, we inactivated the locus in mice. Homozygous Prox1-null mice die at mid-gestation from multiple developmental defects; here we describe the specific effect on lens development. Prox1 inactivation causes abnormal cellular proliferation, downregulated expression of the cell-cycle inhibitors Cdkn1b (also known as p27KIP1) and Cdkn1c (also known as p57KIP2), misexpression of E-cadherin and inappropriate apoptosis. Consequently, mutant lens cells fail to polarize and elongate properly, resulting in a hollow lens. Our data provide evidence that the progression of terminal fibre differentiation and elongation is dependent on Prox1 activity during lens development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Oliver, G. et al. Prox1, a prospero-related homeobox gene expressed during mouse development. Mech. Dev. 44, 3–16 (1993).
Zhang, P. et al. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature 387, 151–158 (1997).
Zhang, P. et al. Cooperation between the Cdk inhibitors p27KIP1 and p57KIP2 in the control of tissue growth and development. Genes Dev. 12, 3162–3167 (1998).
Nishiguchi, S., Wood, H., Kondoh, H., Lovell-Badge, R. & Episkopou, V. Sox1 directly regulates the γ-crystallin genes and is essential for lens development in mice. Genes Dev. 12, 776–781 (1998).
Goring, D.R., Breitman, M.L. & Tsui, L.C. Temporal regulation of six crystallin transcripts during mouse lens development. Exp. Eye Res. 54, 785–795 (1992).
Cartier, M., Breitman, M.L. & Tsui, L.C. A frameshift mutation in the γ-E crystallin gene of the Elo mouse. Nature Genet. 2, 42–45 (1992).
Sandilands, A. et al. Vimentin and CP49/filensin form distinct networks in the lens which are independently modulated during lens fibre cell differentiation. J. Cell Sci. 108, 1397– 1406 (1995).
Takeichi, M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development 102, 639–655 (1988).
Lang, R.A. Apoptosis in mammalian eye development: lens morphogenesis, vascular regression and immune privilege. Cell Death Differ. 4, 12–20 (1997).
Bober, E., Franz, T., Arnold, H.H., Gruss, P. & Tremblay, P. Pax3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development 120, 603– 612 (1994).
Kessel, M. & Gruss, P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell 67, 89–104 (1991).
Gavrieli, Y., Sherman, Y. & Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation J. Cell Biol. 119, 493–501 (1992).
Acknowledgements
We thank M. Torres, S. Geisendorf, S. Mahsur and C. Nagy for help with the initial steps of the knockout; H. Brinkman for technical assistance; R. Lovell-Badge for Sox1 antibody; R. Quinlan for CP49 and filensin antibodies; S.J. Elledge for communicating results before publication; P. Overbeek for suggestions, comments and the Crya1 and Cdkn1c probes; an anonymous referee for helpful comments; and J. Cleveland, G. Grosveld and C. Wright for critical reading of the manuscript. This work was partially supported by National Institutes of Health grant GM58462 (G.O.), by Cancer Center Support (CORE) grant CA 21765 and by the American Lebanese and Syrian Associated Charities (ALSAC) of St. Jude Children's Research Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wigle, J., Chowdhury, K., Gruss, P. et al. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet 21, 318–322 (1999). https://doi.org/10.1038/6844
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/6844
This article is cited by
-
Dynamic changes in whole genome DNA methylation, chromatin and gene expression during mouse lens differentiation
Epigenetics & Chromatin (2023)
-
A Prox1 enhancer represses haematopoiesis in the lymphatic vasculature
Nature (2023)
-
Nicotinamide improves in vitro lens regeneration in a mouse capsular bag model
Stem Cell Research & Therapy (2022)
-
Deletion of the transcription factor Prox-1 specifically in the renal distal convoluted tubule causes hypomagnesemia via reduced expression of TRPM6 and NCC
Pflügers Archiv - European Journal of Physiology (2021)
-
Prox1 inhibits neurite outgrowth during central nervous system development
Cellular and Molecular Life Sciences (2021)