Abstract
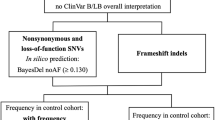
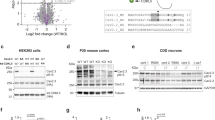
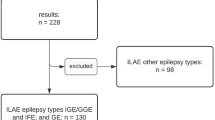
Idiopathic generalized epilepsy (IGE) is an inherited neurological disorder affecting about 0.4% of the world's population. Mutations in ten genes causing distinct forms of idiopathic epilepsy have been identified so far1,2,3,4,5,6,7, but the genetic basis of many IGE subtypes is still unknown. Here we report a gene associated with the four most common IGE subtypes: childhood and juvenile absence epilepsy (CAE and JAE), juvenile myoclonic epilepsy (JME), and epilepsy with grand mal seizures on awakening (EGMA; ref. 8). We identified three different heterozygous mutations in the chloride-channel gene CLCN2 in three unrelated families with IGE. These mutations result in (i) a premature stop codon (M200fsX231), (ii) an atypical splicing (del74–117) and (iii) a single amino-acid substitution (G715E). All mutations produce functional alterations that provide distinct explanations for their pathogenic phenotypes. M200fsX231 and del74–117 cause a loss of function of ClC-2 channels and are expected to lower the transmembrane chloride gradient essential for GABAergic inhibition. G715E alters voltage-dependent gating, which may cause membrane depolarization and hyperexcitability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Steinlein, O.K. & Noebels J.L. Ion channels and epilepsy in man and mouse. Curr. Opin. Genet. Dev. 10, 286–291 (2000).
Berkovic, S.F. & Scheffer, I.E. Genetics of the epilepsies. Epilepsia 42, Suppl 5 16–23 (2001).
Lerche, H., Jurkat-Rott, K. & Lehmann-Horn, F. Ion channels and epilepsy. Am. J. Med. Genet. 106, 146–159 (2001).
Baulac, S. et al. First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the γ2-subunit gene. Nat. Genet. 28, 46–48 (2001).
Wallace, R.H. et al. Mutant GABAA receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 28, 49–52 (2001).
Kalachikov, S. et al. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat. Genet. 30, 335–341 (2002).
Cossette, P. et al. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat. Genet. 31, 184–189 (2002).
Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 30, 389–399 (1989).
Sander, T. et al. Genome search for susceptibility loci of common idiopathic generalized epilepsies. Hum. Mol. Genet. 9, 1465–1472 (2000).
Thiemann, A., Gründer, S., Pusch, M. & Jentsch, T.J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature 356, 57–60 (1992).
Cid, L.P., Montrose-Rafizadeh, C., Smith, D.I., Guggino, W.B. & Cutting, G.R. Cloning of a putative human voltage-gated chloride channel (ClC-2) cDNA widely expressed in human tissues. Hum. Mol. Genet. 4, 407–413 (1995).
Sik, A., Smith, R.L. & Freund, T.F. Distribution of chloride channel-2-immunoreactive neuronal and astrocytic processes in the hippocampus. Neuroscience 101, 51–65 (2000).
Staley, K.J. The role of an inwardly rectifying chloride conductance in postsynaptic inhibition. J. Neurophysiol. 72, 273–284 (1994).
Mladinic, M., Becchetti, A., Didelon, F., Bradbury, A. & Cherubini, E. Low expression of the ClC-2 chloride channel during postnatal development: a mechanism for the paradoxical depolarizing action of GABA and glycine in the hippocampus. Proc. R. Soc. Lond. B Biol. Sci. 266, 1207–1213 (1999).
Staley, K.J., Smith, R., Schaack, J., Wilcox, C. & Jentsch, T.J. Alteration of GABAA receptor function following gene transfer of the ClC-2 chloride channel. Neuron 17, 543–551 (1996).
Fahlke, C., Yu, H.T., Beck, C.L., Rhodes, T.H. & George, A.L. Jr. Pore-forming segments in voltage-gated chloride channels. Nature 390, 529–532 (1997).
Dutzler, R., Campbell, E.B., Cadene, M., Chait, B.T. & MacKinnon, R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415, 287–294 (2002).
Miller, C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos. Trans. R. Soc. Lond. B Biol. Sci. 299, 401–411 (1982).
Thompson, S.M. & Gähwiller, B.H. Activity-dependent disinhibition II: Effects of extracellular potassium, furosemide, and membrane potential on ECl in hippocampal CA3 neurons. J. Neurophysiol. 61, 512–523 (1989).
Misgeld, U., Deisz, R.A., Dodt, H.U. & Lux, H.D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science 232, 1413–1415 (1986).
Rivera, C. et al. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255 (1999).
Kaila, K., Lamsa, K., Smirnov, S., Taira, T. & Voipio, J. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J. Neurosci. 17, 7662–7672 (1997).
Payne, J.A. Functional characterization of the neuronal-specific K–Cl cotransporter: implications for [K+]o regulation. Am. J. Physiol. 273, C1516–C1525 (1997).
Bösl, M.R. et al. Male germ cells and photoreceptors, both dependent on close cell-cell interactions, degenerate upon ClC-2 Cl− channel disruption. EMBO J. 20, 1289–1299 (2001).
Ross, S.A. et al. Phenotypic characterization of an α4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J. Neurosci. 20, 6431–6441 (2000).
Labarca, C. et al. Point mutant mice with hypersensitive α4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc. Natl. Acad. Sci. USA 98, 2786–2791 (2001).
Serratosa, J.M. Idiopathic epilepsies with a complex mode of inheritance. Epilepsia 40, 12–16 (1999)
Ma, D. et al. Role of ER export signals in controlling surface potassium channel numbers. Science 291, 316–319 (2001).
Fahlke, C., Knittle, T., Gurnett, C.A., Campbell, K.P. & George, A.L. Jr. Subunit stoichiometry of human muscle chloride channels. J. Gen. Physiol. 109, 93–104 (1997).
Cui, J., Cox, D.H. & Aldrich, R.W. Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca2+-activated K+ channels. J. Gen. Physiol. 109, 647–673 (1997).
Acknowledgements
The authors thank all affected individuals and their families who participated in this study; H. Beck, F. Lehmann-Horn, J.P. Johnson and A. Ryan for discussion on the manuscript; P.C. Heinrich for support; and G. Cutting for providing the pBKRSV-hClC-2 construct. This work was supported by the German Volkswagen-Stiftung (to A.H.), the Deutsche Forschungs-Gemeinschaft (to A.H., C.F., H.L. & T.S.), the German Bundesministerium für Bildung und Forschung (to A.H. & H.L.), the Stiftung Michael (to T.S.), BONFOR (to A.H.) and the Muscular Dystrophy Association (to C.F.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Haug, K., Warnstedt, M., Alekov, A. et al. Mutations in CLCN2 encoding a voltage-gated chloride channel are associated with idiopathic generalized epilepsies. Nat Genet 33, 527–532 (2003). https://doi.org/10.1038/ng1121
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ng1121
This article is cited by
-
The Molecular Biology of Genetic-Based Epilepsies
Molecular Neurobiology (2014)
-
An Association Analysis of Reelin Gene (RELN) Polymorphisms with Childhood Epilepsy in Eastern Indian Population from West Bengal
Cellular and Molecular Neurobiology (2011)
-
No evidence for a role of CLCN2 variants in idiopathic generalized epilepsy
Nature Genetics (2010)
-
Mutations affecting GABAergic signaling in seizures and epilepsy
Pflügers Archiv - European Journal of Physiology (2010)
-
Genome wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) Heynh and Oryza sativa L. reveals their developmental and stress regulation
BMC Genomics (2009)