Abstract
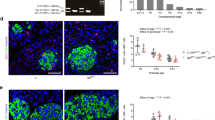
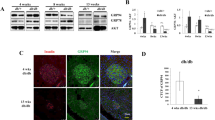
Regulation of glucose homeostasis by insulin depends on the maintenance of normal β-cell mass and function. Insulin-like growth factor 1 (Igf1) has been implicated in islet development and differentiated function1,2, but the factors controlling this process are poorly understood. Pancreatic islets produce Igf1 and Igf2, which bind to specific receptors on β-cells3,4,5,6. Igf1 has been shown to influence β-cell apoptosis7, and both Igf1 and Igf2 increase islet growth8,9; Igf2 does so in a manner additive with fibroblast growth factor 2 (ref. 10). When mice deficient for the Igf1 receptor (Igf1r+/− ) are bred with mice lacking insulin receptor substrate 2 (Irs2−/−), the resulting compound knockout mice show a reduction in mass of β-cells11 similar to that observed in pancreas of Igf1r−/− mice (ref. 11), suggesting a role for Igf1r in growth of β-cells. It is possible, however, that the effects in these mice occur secondary to changes in vascular endothelium12 or in the pancreatic ductal cells, or because of a decrease in the effects of other hormones implicated in islet growth. To directly define the role of Igf1, we have created a mouse with a β-cell–specific knockout of Igf1r (βIgf1r−/−). These mice show normal growth and development of β-cells, but have reduced expression of Slc2a2 (also known as Glut2) and Gck (encoding glucokinase) in β-cells, which results in defective glucose-stimulated insulin secretion and impaired glucose tolerance. Thus, Igf1r is not crucial for islet β-cell development, but participates in control of differentiated function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bonner-Weir, S. & Smith, F.E. Islet cell growth and the growth factors involved. Trends Endocrinol. Metab. 5, 60–64 (1994).
Swenne, I. Pancreatic β-cell growth and diabetes mellitus. Diabetologia 35, 193–201 (1992).
Blakesley, V.A., Butler, A.A., Koval, A.P., Okubo, Y. & LeRoith, D. in The IGF System 143–164 (Humana, New Jersey, 1999).
De Meyts, P. et al. The insulin-like growth factor-I receptor structure, ligand-binding mechanism and signal transduction. Horm. Res. 42, 152–169 (1994).
Van Schravendijk, C.F., Foriers, A., Van den Brande, J.L. & Pipeleers, D.G. Evidence for the presence of type I insulin-like growth factor receptors on rat pancreatic A and B cells. Endocrinology 121, 1784–1788 (1987).
Zhang, Q. et al. Insulin-like growth factor II signaling through the insulin-like growth factor II/mannose-6-phosphate receptor promotes exocytosis in insulin-secreting cells. Proc. Natl Acad. Sci. USA 94, 6232–6237 (1997).
Rhodes, C.J. IGF1 and GH post-receptor signaling mechanisms for pancreatic β-cell replication. J. Mol. Endocrinol. 24, 303–311 (2000).
Otonkoski, T., Knip, M., Wong, I. & Simell, O. Effects of growth hormone and insulin-like growth factor I on endocrine function of human fetal islet-like cell clusters during long-term tissue culture. Diabetes 37, 1678–1683 (1988).
Rabinovitch, A., Quigley, C., Russell, T., Patel, Y. & Mintz, D.H. Insulin and multiplication stimulating activity (an insulin-like growth factor) stimulate islet β-cell replication in neonatal rat pancreatic monolayer culutes. Diabetes 31, 160–164 (1982).
Arany, E. & Hill, D.J. Ontogeny of fibroblast growth factors in the early development of the rat endocrine pancreas. Pediatr. Res. 48, 389–403 (2000).
Withers, D.J. et al. IRS-2 coordinates Igf1r-mediated β cell development and peripheral insulin signaling. Nature Genet. 23, 32–40 (1999).
Lammert, E., Cleaver, O. & Melton, D. Induction of pancreatic differentiation by signals from blood vessels. Science 294, 564–567 (2001).
Holzenberger, M. et al. A targeted partial invalidation of the Igf1r gene in mice causes a postnatal growth deficit. Endocrinology 141, 2557–2566 (2000).
Kulkarni, R.N. et al. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96, 329–339 (1999).
Leahy, J.L. & Vandekerkhove, K.M. Insulin-like growth factor 1 at physiological concentrations is a potent inhibitor of insulin secretion. Endocrinology 126, 1593–1598 (1990).
Duncan, S.A., Angeles Navas, M., Dufort, D., Rossant, J. & Stoffel, M. Regulation of a transcription factor network required for differentiation and metabolism. Science 281, 692–695 (1998).
Argetsinger, L.S., Norstedt, G., Billestrup, N., White, M.F. & Carter-Su, C. Growth hormone, interferon-gamma, and leukemia inhibitory factor utilize insulin receptor substrate 2 in intracellular signaling. J. Biol. Chem. 271, 29415–29421 (1996).
Bole-Feysot, C., Goffin, V., Edery, M., Binart, N. & Kelly, P.A. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 19, 225–268 (1998).
Sanford, L.P., Kallapur, S., Ormsby, I. & Doetschman, T. Influence of genetic background on knockout mouse phenotypes. in Gene Knockout Protocols 217–226 (Humana, Totowa, 2001).
Gannon, M., Shiota, C., Postic, C., Wright, C.V.E. & Magnuson, M. Analysis of the cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis 26, 139–141 (2000).
Nguyen, H.Q. et al. Expression of keratinocyte growth factor in embryonic liver of transgenic mice causes changes in epithelial growth and differentiation resuling in polycystic kidneys and other organ malformation. Oncogene 12, 2109–2119 (1996).
Kulkarni, R.N. & Kahn, C.R. Genetic models of insulin resistance: alterations in β-cell biology. in Molecular Basis of Pancreas Development and Function 299–323 (Kluwer Academic, New York, 2001).
Smith, F.E., Rosen, K.M., Villa, K.L., Weir, G.C. & Bonner, W.S. Enhanced insulin-like growth factor I gene expression in regenerating rat pancreas. Proc. Natl Acad. Sci. USA 88, 6152–6156 (1991).
Aspinwall, C.A. et al. Role of insulin receptor substrate-1, phosphatidylinositol 3-kinase, and release of intracellular Ca2+ stores in insulin-stimulated insulin secretion in β-cell. J. Biol. Chem. 275, 22331–22338 (2000).
Leibiger, I.B., Leibiger, B., Moede, T. & Berggren, P.O. Exocytosis of insulin promotes insulin gene transcription via the insulin receptor/PI-3 kinase/p70 s6 kinase and CaM kinase. Mol. Cell 1, 933–938 (1998).
Leibiger, B. et al. Selective insulin signaling through a and b insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic β cells. Mol. Cell 7, 559–570 (2001).
Kulkarni, R.N. et al. Altered function of insulin receptor substrate 1-deficient mouse islets and cultured β-cell lines. J. Clin. Invest. 104, R69–R75 (1999).
da Silva Xavier, G., Varadi, A., Ainscow, E.K. & Rutter, G.A. Regulation of gene expresion by glucose in pancreatic β-cells (MIN6) via insulin secretion and activation of phosphatidylinositol 3′-kinase. J. Biol. Chem. 275, 36269–36277 (2000).
Wilson, P.A. & Melton, D.A. Mesodermal patterning by an inducer gradient depends on secondary cell-cell communication. Curr. Biol. 4, 676–686 (1994).
Acknowledgements
We thank R.L. Quinn and E. Fletcher for assistance with animal care, and J. Marr for secretarial assistance. We thank K.C. Hayes and staff at the Brandeis Animal Facility (Waltham) for housing and maintenance of the mouse colonies. This study was supported by a Clinician Scientist Development Award from the National Institutes of Health (to R.N.K.), grants from the NIH (to C.R.K. and M.S.), the DERC grant (Specialized Assay Core & Advanced Microscopy Core, Joslin Diabetes Center) and a grant from the Juvenile Diabetes Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kulkarni, R., Holzenberger, M., Shih, D. et al. β-cell–specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter β-cell mass. Nat Genet 31, 111–115 (2002). https://doi.org/10.1038/ng872
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ng872
This article is cited by
-
Targeting pancreatic β cells for diabetes treatment
Nature Metabolism (2022)
-
Insulin-like growth factor 1 receptor mediates photoreceptor neuroprotection
Cell Death & Disease (2022)
-
Mice with gene alterations in the GH and IGF family
Pituitary (2022)
-
New-found brake calibrates insulin action in β-cells
Nature (2021)
-
PDX1LOW MAFALOW β-cells contribute to islet function and insulin release
Nature Communications (2021)