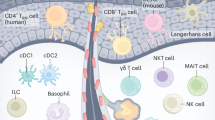
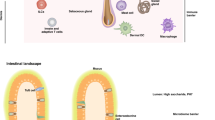
Abstract
The skin is a highly complex organ interspersed with a variety of smaller organ-like structures and a plethora of cell types that together perform essential functions such as physical sensing, temperature control, barrier maintenance and immunity. In this Review, we outline many of the innate and adaptive immune cell types associated with the skin, focusing on the steady state in mice and men, and include a broad update of dendritic cell function and T cell surveillance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Grice, E.A. & Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 9, 244–253 (2011).
Cavassani, K.A. et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J. Exp. Med. 205, 2609–2621 (2008).
Lai, Y. et al. Commensal bacteria regulate Toll-like receptor 3–dependent inflammation after skin injury. Nat. Med. 15, 1377–1382 (2009).
Naik, S. et al. Compartmentalized control of skin Immunity by resident commensals. Science 337, 1115–1119 (2012).
Nestle, F.O., Di Meglio, P., Qin, J.-Z. & Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 (2009).
Kubo, A., Nagao, K., Yokouchi, M., Sasaki, H. & Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 206, 2937–2946 (2009).
Kuo, I.-H., Yoshida, T., De Benedetto, A. & Beck, L.A. The cutaneous innate immune response in patients with atopic dermatitis. J. Allergy Clin. Immunol. 131, 266–278 (2013).
Brown, S.J. & McLean, W.H.I. One remarkable molecule: filaggrin. J. Invest. Dermatol. 132, 751–762 (2012).
Meephansan, J., Tsuda, H., Komine, M., Tominaga, S.-i. & Ohtsuki, M. Regulation of IL-33 expression by IFN-γ and tumor necrosis factor-α in normal human epidermal keratinocytes. J. Invest. Dermatol. 132, 2593–2600 (2012).
Nagao, K. et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 13, 744–752 (2012).This study provided important insight into production of chemokines by different regions of hair follicles and implicated some of these in the recruitment of monocytes and Langerhans cells into the epidermis. This work raised the idea that hair follicles may act as a gateway to the epidermis.
Zhu, J., Yamane, H. & Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010).
Gebhardt, T. et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10, 524–530 (2009).Described is a non-recirculating form of tissue-resident memory CD8+ T cells, which was the first evidence that these cells could provide local protection against infection.
Clark, R.A. et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 (2006).
Zhu, J. et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 497, 494–497 (2013).
MacLeod, A.S. & Havran, W.L. Functions of skin-resident γδ T cells. Cell Mol. Life Sci. 68, 2399–2408 (2011).
Toulon, A. et al. A role for human skin-resident T cells in wound healing. J. Exp. Med. 206, 743–750 (2009).
Maricich, S.M. et al. Merkel cells are essential for light-touch responses. Science 324, 1580–1582 (2009).
Schneider, M.R., Schmidt-Ullrich, R. & Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 19, R132–R142 (2009).
Paus, R. & Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 341, 491–497 (1999).
Ito, M. et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 (2007).
Chodaczek, G., Papanna, V., Zal, M.A. & Zal, T. Body-barrier surveillance by epidermal γδ TCRs. Nat. Immunol. 13, 272–282 (2012).This work provided evidence that γδ T cells of the epidermis (referred to as dendritic epidermal T cells or DETCs) are constitutively signaled through their TCR via polarized interactions anchored in epidermal regions associated with tight junctions. These interactions are thought to monitor steady-state integrity of the epidermis. This report also provides some outstanding long-term live imaging of DETCs, showing their dendrite orientation and cellular replication.
Sumaria, N. et al. Cutaneous immunosurveillance by self-renewing dermal γδ T cells. J. Exp. Med. 208, 505–518 (2011).
Ye, S.K. et al. Differential roles of cytokine receptors in the development of epidermal gamma delta T cells. J. Immunol. 167, 1929–1934 (2001).
De Creus, A. et al. Developmental and functional defects of thymic and epidermal V gamma 3 cells in IL-15–deficient and IFN regulatory factor-1–deficient mice. J. Immunol. 168, 6486–6493 (2002).
Hayday, A.C. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity 31, 184–196 (2009).
Girardi, M. et al. Regulation of cutaneous malignancy by gammadelta T cells. Science 294, 605–609 (2001).
Witherden, D.A. et al. The Junctional Adhesion Molecule JAML Is a Costimulatory Receptor for Epithelial T Cell Activation. Science 329, 1205–1210 (2010).
Witherden, D.A. et al. The CD100 receptor interacts with its plexin B2 ligand to regulate epidermal γδ T cell function. Immunity 37, 314–325 (2012).
Havran, W.L., Chien, Y.H. & Allison, J.P. Recognition of self antigens by skin-derived T cells with invariant gamma delta antigen receptors. Science 252, 1430–1432 (1991).
Jameson, J.M., Cauvi, G., Sharp, L.L., Witherden, D.A. & Havran, W.L. γδ T cell-induced hyaluronan production by epithelial cells regulates inflammation. J. Exp. Med. 201, 1269–1279 (2005).
Boismenu, R., Feng, L., Xia, Y.Y., Chang, J.C. & Havran, W.L. Chemokine expression by intraepithelial γδ T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J. Immunol. 157, 985–992 (1996).
Matsue, H., Cruz, P.D., Bergstresser, P.R. & Takashima, A. Profiles of cytokine mRNA expressed by dendritic epidermal T cells in mice. J. Invest. Dermatol. 101, 537–542 (1993).
Strid, J., Sobolev, O., Zafirova, B., Polic, B. & Hayday, A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science 334, 1293–1297 (2011).
Gray, E.E. et al. Deficiency in IL-17–committed Vγ4+ γδ T cells in a spontaneous Sox13-mutant CD45.1+ congenic mouse substrain provides protection from dermatitis. Nat. Immunol. 14, 584–592 (2013).
Pantelyushin, S. et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 122, 2252–2256 (2012).
Cai, Y. et al. Pivotal role of dermal IL-17–producing γδ T cells in skin inflammation. Immunity 35, 596–610 (2011).
Philip, N.H. & Artis, D. New friendships and old feuds: relationships between innate lymphoid cells and microbial communities. Immunol. Cell Biol. 91, 225–231 (2013).
Kim, B.S. et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 5, 170ra116 (2013).
Roediger, B. et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 14, 564–573 (2013).
Luci, C. et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10, 75–82 (2009).
Batista, M.D. et al. Skewed distribution of natural killer cells in psoriasis skin lesions. Exp. Dermatol. 22, 64–66 (2013).
Ebert, L.M., Meuter, S. & Moser, B. Homing and function of human skin γδ T cells and NK cells: relevance for tumor surveillance. J. Immunol. 176, 4331–4336 (2006).
Minty, A. et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature 362, 248–250 (1993).
Cohn, L. et al. Th2-induced airway mucus production is dependent on IL-4Rα, but not on eosinophils. J. Immunol. 162, 6178–6183 (1999).
Pope, S.M. et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J. Allergy Clin. Immunol. 108, 594–601 (2001).
Segura, E. et al. Differential expression of pathogen-recognition molecules between dendritic cell subsets revealed by plasma membrane proteomic analysis. Mol. Immunol. 47, 1765–1773 (2010).
Edwards, A.D. et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8α+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 33, 827–833 (2003).
Miller, J.C. et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 13, 888–899 (2012).
Belz, G.T. & Nutt, S.L. Transcriptional programming of the dendritic cell network. Nat. Rev. Immunol. 12, 101–113 (2012).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Hoeffel, G. et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac–derived macrophages. J. Exp. Med. 209, 1167–1181 (2012).
Naik, S.H. et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 7, 663–671 (2006).
Ginhoux, F. et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206, 3115–3130 (2009).This report shows that CD103+ DCs exist in many tissues, and provides important information about the origin of these DCs and their CD11b+ counterparts in the same tissues, showing the latter are heterogeneous in origin.
Heath, W.R. & Carbone, F.R. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat. Immunol. 10, 1237–1244 (2009).
Onai, N. et al. A clonogenic progenitor with prominent plasmacytoid dendritic cell developmental potential. Immunity 38, 943–957 (2013).
Jakubzick, C. et al. Blood monocyte subsets differentially give rise to CD103+ and CD103− pulmonary dendritic cell populations. J. Immunol. 180, 3019–3027 (2008).
Ginhoux, F. et al. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7, 265–273 (2006).
Farache, J., Zigmond, E., Shakhar, G. & Jung, S. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunol. Cell Biol. 91, 232–239 (2013).
Serbina, N.V., Salazar-Mather, T.P., Biron, C.A., Kuziel, W.A. & Pamer, E.G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70 (2003).
Wakim, L.M., Waithman, J., van Rooijen, N., Heath, W.R. & Carbone, F.R. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008).
Henri, S. et al. Disentangling the complexity of the skin dendritic cell network. Immunol. Cell Biol. 88, 366–375 (2010).
Henri, S. et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 207, 189–206 (2010).
Haniffa, M. et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 37, 60–73 (2012).This report identifies the human skin counterpart of the mouse CD103+ dermal DCs.
Poulin, L.F. et al. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J. Exp. Med. 204, 3119–3131 (2007).
Bursch, L.S. et al. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 204, 3147–3156 (2007).
Ginhoux, F. et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 204, 3133–3146 (2007).
Shortman, K. & Heath, W.R. The CD8+ dendritic cell subset. Immunol. Rev. 234, 18–31 (2010).
Zhang, J.-G. et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity 36, 646–657 (2012).
Ahrens, S. et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 36, 635–645 (2012).
Bedoui, S. et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10, 488–495 (2009).
Schlitzer, A. et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983 (2013).
Persson, E.K. et al. IRF4 transcription-factor-dependent CD103+CD11b+ dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 38, 958–969 (2013).
Itano, A.A. & Jenkins, M.K. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4, 733–739 (2003).
McLachlan, J.B., Catron, D.M., Moon, J.J. & Jenkins, M.K. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity 30, 277–288 (2009).
Ritter, U., Meissner, A., Scheidig, C. & Korner, H. CD8α- and Langerin-negative dendritic cells, but not Langerhans cells, act as principal antigen-presenting cells in leishmaniasis. Eur. J. Immunol. 34, 1542–1550 (2004).
Guilliams, M. et al. Skin-draining lymph nodes contain dermis-derived CD103− dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood 115, 1958–1968 (2010).
Coombes, J.L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF- and retinoic acid dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Ouchi, T. et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J. Exp. Med. 208, 2607–2613 (2011).
Kaplan, D.H. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 31, 446–451 (2010).
Romani, N., Brunner, P.M. & Stingl, G. Changing views of the role of Langerhans cells. J. Invest. Dermatol. 132, 872–881 (2012).
Igyarto, B.Z. & Kaplan, D.H. Antigen presentation by Langerhans cells. Curr. Opin. Immunol. 25, 115–119 (2013).
Allan, R.S. et al. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 301, 1925–1928 (2003).
Kaplan, D.H., Jenison, M.C., Saeland, S., Shlomchik, W.D. & Shlomchik, M.J. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 23, 611–620 (2005).
Igyarto, B.Z. et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272 (2011).This report is one of the first to provide convincing evidence that Langerhans cells have a function in antigen presentation and priming of T cells to infectious agents. It provides evidence that Langerhans cells induce T H 17 cell–mediated immunity to C. albicans . It also shows that Langerin+ (CD103+) DCs are important for T H 1 cell and cytotoxic T cell responses.
Haley, K. et al. Langerhans cells require MyD88-dependent signals for Candida albicans response but not for contact hypersensitivity or migration. J. Immunol. 188, 4334–4339 (2012).
Shklovskaya, E. et al. Langerhans cells are precommitted to immune tolerance induction. Proc. Natl. Acad. Sci. USA 108, 18049–18054 (2011).
Kautz-Neu, K. et al. Langerhans cells are negative regulators of the anti-Leishmania response. J. Exp. Med. 208, 885–891 (2011).
Seneschal, J., Clark, R.A., Gehad, A., Baecher-Allan, C.M. & Kupper, T.S. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity 36, 873–884 (2012).
Villadangos, J.A. & Young, L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 29, 352–361 (2008).
Nestle, F.O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 202, 135–143 (2005).
Banchereau, J., Pascual, V. & Type, I. Interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383–392 (2006).
Di Meglio, P., Perera, G.K. & Nestle, F.O. The multitasking organ: recent insights into skin immune function. Immunity 35, 857–869 (2011).
Iezzi, G. et al. Lymph node resident rather than skin-derived dendritic cells initiate specific T cell responses after Leishmania major infection. J. Immunol. 177, 1250–1256 (2006).
Itano, A.A. et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Allan, R.S. et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25, 153–162 (2006).
Boyman, O. et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J. Exp. Med. 199, 731–736 (2004).
Gebhardt, T. et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219 (2011).Provides clear evidence that CD8+ T cells do not form a recirulating memory cell population that traffics through the skin and instead form resident memory cells in the epidermis. This contrasts CD4+ memory T cells, which do form a recirculating population that traffics through the dermis, lymph nodes, spleen and blood. These recirculating CD4+ memory T cells are shown to have skin tropism.
Teijaro, J.R. et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187, 5510–5514 (2011).
Anderson, K.G. et al. Cutting Edge: Intravascular Staining Redefines Lung CD8 T Cell Responses. J. Immunol. 189, 2702–2706 (2012).
Masopust, D. et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 (2010).
Casey, K.A. et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 188, 4866–4875 (2012).
Hofmann, M. & Pircher, H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc. Natl. Acad. Sci. USA 108, 16741–16746 (2011).
Schenkel, J.M., Fraser, K.A., Vezys, V. & Masopust, D. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol. 14, 509–513 (2013).This work shows that tissue-resident memory T cells can recruit circulating memory T cells in response to antigen detection. Thus, the ability of T RM cells to control infection is likely mediated both by their own effector function and their ability to recruit additional circulating effectors.
Klonowski, K.D. et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20, 551–562 (2004).
Mackay, L.K. et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. USA 109, 7037–7042 (2012).This study was the first to show that T RM cells can be seeded into tissues (skin and vagina) by inflammation and that this can lead to protection against infection.
Wakim, L.M., Woodward-Davis, A. & Bevan, M.J. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. USA 107, 17872–17879 (2010).
Clark, R.A. et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Science Translational Medicine 4, 117ra117 (2012).This work provides important evidence that human skin may contain non-recirculating T cells, that is, tissue-resident memory T cells, by demonstrating the presence of T cells in skin of patients that have had their circulating T cells depleted by treatment with monoclonal antibody.
Jiang, X. et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483, 227–231 (2012).
Bromley, S.K., Yan, S., Tomura, M., Kanagawa, O. & Luster, A.D. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J. Immunol. 190, 970–976 (2013).
Bromley, S.K., Thomas, S.Y. & Luster, A.D. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6, 895–901 (2005).
Debes, G.F. et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 6, 889–894 (2005).
Wald, A. et al. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J. Clin. Invest. 99, 1092–1097 (1997).
Shin, H. & Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467 (2012).
Lai, Y. et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J. Invest. Dermatol. 130, 2211–2221 (2010).
Acknowledgements
We thank S. Mueller and A. Zaid for the immunohistology in Figure 2b,c. Supported by the Australian Research Council (W.R.H.) and the National Health and Medical Research Council of Australia (W.R.H. and F.R.C.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Heath, W., Carbone, F. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol 14, 978–985 (2013). https://doi.org/10.1038/ni.2680
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ni.2680
This article is cited by
-
Key factors to establish the ovalbumin-induced atopic dermatitis minipig model: age and body weight
Laboratory Animal Research (2022)
-
Tissue-specific immunity in helminth infections
Mucosal Immunology (2022)
-
Nociceptive sensory neurons promote CD8 T cell responses to HSV-1 infection
Nature Communications (2021)
-
Dendritic cell migration in inflammation and immunity
Cellular & Molecular Immunology (2021)
-
Molecular and histopathological profiling of imiquimod induced dermatosis in Swiss Wistar rats: contribution to the rat model for novel anti-psoriasis treatments
Molecular Biology Reports (2021)