Abstract
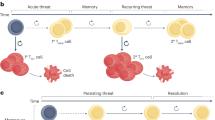
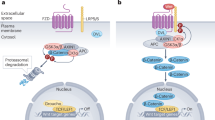
Self-renewing cell populations such as hematopoietic stem cells and memory B and T lymphocytes might be regulated by shared signaling pathways1. The Wnt–β-catenin pathway is an evolutionarily conserved pathway that promotes hematopoietic stem cell self-renewal and multipotency by limiting stem cell proliferation and differentiation2,3, but its role in the generation and maintenance of memory T cells is unknown. We found that induction of Wnt–β-catenin signaling by inhibitors of glycogen sythase kinase-3β or the Wnt protein family member Wnt3a arrested CD8+ T cell development into effector cells. By blocking T cell differentiation, Wnt signaling promoted the generation of CD44lowCD62LhighSca-1highCD122highBcl-2high self-renewing multipotent CD8+ memory stem cells with proliferative and antitumor capacities exceeding those of central and effector memory T cell subsets. These findings reveal a key role for Wnt signaling in the maintenance of 'stemness' in mature memory CD8+ T cells and have major implications for the design of new vaccination strategies and adoptive immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Luckey, C.J. et al. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 103, 3304–3309 (2006).
Staal, F.J., Luis, T.C. & Tiemessen, M.M. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 8, 581–593 (2008).
Fleming, H.E. et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2, 274–283 (2008).
Verbeek, S. et al. An HMG-box-containing T-cell factor required for Thymocyte differentiation. Nature 374, 70–74 (1995).
Jeannet, G. et al. Long-term, multilineage hematopoiesis occurs in the combined absence of β-catenin and γ-catenin. Blood 111, 142–149 (2008).
Schilham, M.W. et al. Critical involvement of Tcf-1 in expansion of Thymocytes. J. Immunol. 161, 3984–3991 (1998).
Willinger, T. et al. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J. Immunol. 176, 1439–1446 (2006).
Hinrichs, C.S. et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 111, 5326–5333 (2008).
Williams, M.A., Ravkov, E.V. & Bevan, M.J. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity 28, 533–545 (2008).
Ding, S. et al. Synthetic small molecules that control stem cell fate. Proc. Natl. Acad. Sci. USA 100, 7632–7637 (2003).
Roose, J. et al. Synergy between tumor suppressor APC and the β-catenin-Tcf4 target Tcf1. Science 285, 1923–1926 (1999).
Hovanes, K et al. β-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat. Genet. 28, 53–57 (2001).
Mann, B. et al. Target genes of β-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. USA 96, 1603–1608 (1999).
Katoh, M. & Katoh, M. Comparative integromics on FZD7 orthologs: conserved binding sites for PU.1, SP1, CCAAT-box and TCF/LEF/SOX transcription factors within 5′-promoter region of mammalian FZD7 orthologs. Int. J. Mol. Med. 19, 529–533 (2007).
Zeng, Y.A. & Verheyen, E.M. Nemo is an inducible antagonist of Wingless signaling during Drosophila wing development. Development 131, 2911–2920 (2004).
Overwijk, W.W. et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 198, 569–580 (2003).
Gattinoni, L. et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 115, 1616–1626 (2005).
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3–specific inhibitor. Nat. Med. 10, 55–63 (2004).
Meijer, L., Flajolet, M. & Greengard, P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol. Sci. 25, 471–480 (2004).
Ohteki, T. et al. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. J. Exp. Med. 192, 99–104 (2000).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003).
Pantaleo, G. & Harari, A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat. Rev. Immunol. 6, 417–423 (2006).
Pearce, E.L. et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302, 1041–1043 (2003).
Appay, V., Douek, D.C. & Price, D.A. CD8+ T cell efficacy in vaccination and disease. Nat. Med. 14, 623–628 (2008).
Zhang, Y., Joe, G., Hexner, E., Zhu, J. & Emerson, S.G. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat. Med. 11, 1299–1305 (2005).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Kaech, S.M. & Wherry, E.J. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27, 393–405 (2007).
Klebanoff, C.A., Gattinoni, L. & Restifo, N.P. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol. Rev. 211, 214–224 (2006).
Tough, D.F. & Sprent, J. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179, 1127–1135 (1994).
Surh, C.D. & Sprent, J. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J. Exp. Med. 192, F9–F14 (2000).
Murali-Krishna, K. et al. Persistence of memory CD8 T cells in MHC class I–deficient mice. Science 286, 1377–1381 (1999).
Wrzesinski, C. et al. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J. Clin. Invest. 117, 492–501 (2007).
Klebanoff, C.A. et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. USA 102, 9571–9576 (2005).
Wherry, E.J. et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4, 225–234 (2003).
Gattinoni, L., Powell, D.J. Jr., Rosenberg, S.A. & Restifo, N.P. Adoptive immunotherapy for cancer: building on success. Nat. Rev. Immunol. 6, 383–393 (2006).
Morgan, R.A. et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006).
Fearon, D.T., Manders, P. & Wagner, S.D. Arrested differentiation, the self-renewing memory lymphocyte and vaccination. Science 293, 248–250 (2001).
Ding, Y., Shen, S., Lino, A.C., Curotto de Lafaille, M.A. & Lafaille, J.J. β-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat. Med. 14, 162–169 (2008).
Chang, J.T. et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315, 1687–1691 (2007).
Mizumoto, K. & Sawa, H. Two βs or not two βs: regulation of asymmetric division by β-catenin. Trends Cell Biol. 17, 465–473 (2007).
Acknowledgements
This research was supported by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Center for Cancer Research. We would like to thank S.A. Rosenberg, C.A. Klebanoff and S. Kerkar for critical review of the manuscript and A. Mixon and S. Farid of the Flow Cytometry Unit for Flow Cytometry analyses and sorting. This study was done in partial fulfillment of a PhD in Biochemistry (to D.C.P.) at the George Washington University, Washington, DC.
Author information
Authors and Affiliations
Contributions
L.G. designed, performed and analyzed experiments and wrote the paper; X.-S.Z. D.C.P., Y.J., C.S.H., Z.Y., C.W., A.B., L.C., L.M.G., C.M.P. and P.M. performed experiments; and N.P.R. designed experiments and wrote the paper.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–8 (PDF 832 kb)
Rights and permissions
About this article
Cite this article
Gattinoni, L., Zhong, XS., Palmer, D. et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med 15, 808–813 (2009). https://doi.org/10.1038/nm.1982
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nm.1982
This article is cited by
-
IL-10-expressing CAR T cells resist dysfunction and mediate durable clearance of solid tumors and metastases
Nature Biotechnology (2024)
-
Multifaceted effects of LRP6 in cancer: exploring tumor development, immune modulation and targeted therapies
Medical Oncology (2024)
-
Eribulin promotes proliferation of CD8+ T cells and potentiates T cell-mediated anti-tumor activity against triple-negative breast cancer cells
Breast Cancer Research and Treatment (2024)
-
New insights into the stemness of adoptively transferred T cells by γc family cytokines
Cell Communication and Signaling (2023)
-
Harnessing CD3 diversity to optimize CAR T cells
Nature Immunology (2023)