Abstract
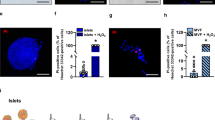
In type I diabetes mellitus, islet transplantation provides a moment-to-moment fine regulation of insulin. Success rates vary widely, however, necessitating suitable methods to monitor islet delivery, engraftment and survival. Here magnetic resonance–trackable magnetocapsules have been used simultaneously to immunoprotect pancreatic β-cells and to monitor, non-invasively in real-time, hepatic delivery and engraftment by magnetic resonance imaging (MRI). Magnetocapsules were detected as single capsules with an altered magnetic resonance appearance on capsule rupture. Magnetocapsules were functional in vivo because mouse β-cells restored normal glycemia in streptozotocin-induced diabetic mice and human islets induced sustained C-peptide levels in swine. In this large-animal model, magnetocapsules could be precisely targeted for infusion by using magnetic resonance fluoroscopy, whereas MRI facilitated monitoring of liver engraftment over time. These findings are directly applicable to ongoing improvements in islet cell transplantation for human diabetes, particularly because our magnetocapsules comprise clinically applicable materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shapiro, A.M. et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343, 230–238 (2000).
Ault, A. Edmonton's islet success tough to duplicate elsewhere. Lancet 361, 2054 (2003).
Shapiro, A.M. et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 355, 1318–1330 (2006).
Soon-Shiong, P. et al. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 343, 950–951 (1994).
Zhou, D., Sun, Y.L., Vacek, I., Ma, P. & Sun, A.M. Normalization of diabetes in cynomolgus monkeys by xenotransplantation of microencapsulated porcine islets. Transplant. Proc. 26, 1091 (1994).
Toso, C. et al. Intra-portal injection of 400-micron microcapsules in a large-animal model. Transpl. Int. 16, 405–410 (2003).
Toso, C. et al. Effect of microcapsule composition and short-term immunosuppression on intraportal biocompatibility. Cell Transplant. 14, 159–167 (2005).
Orive, G. et al. Cell encapsulation: promise and progress. Nat. Med. 9, 104–107 (2003).
Jacobson, R.M. & Poland, G.A. Studies of equivalence in clinical vaccine research. Vaccine 23, 2315–2317 (2005).
Stuber, M., Gilson, W.D., Kedziorek, D.A., Bulte, J.W.M. & Kraitchman, D.L. Signal-enhanced visualization of magnetic nanoparticle-labeled stem cells using inversion recovery on-resonant water suppression (IRON). J. Cardiovasc. Magn. Reson. 8, 13–14 (2006).
Elliott, R.B. et al. Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant. Proc. 37, 3505–3508 (2005).
Bulte, J.W. et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat. Biotechnol. 19, 1141–1147 (2001).
de Vries, I.J. et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 23, 1407–1413 (2005).
Kostura, L., Kraitchman, D.L., Mackay, A.M., Pittenger, M.F. & Bulte, J.W. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 17, 513–517 (2004).
Oca-Cossio, J. et al. Magnetically labeled insulin-secreting cells. Biochem. Biophys. Res. Commun. 319, 569–575 (2004).
Kriz, J. et al. Magnetic resonance imaging of pancreatic islets in tolerance and rejection. Transplantation 80, 1596–1603 (2005).
Evgenov, N.V., Medarova, Z., Dai, G., Bonner-Weir, S. & Moore, A. In vivo imaging of islet transplantation. Nat. Med. 12, 144–148 (2006).
Jirak, D. et al. MRI of transplanted pancreatic islets. Magn. Reson. Med. 52, 1228–1233 (2004).
Evgenov, N.V. et al. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes 55, 2419–2428 (2006).
Frank, J.A. et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology 228, 480–487 (2003).
Tai, J.H. et al. Imaging islets labeled with magnetic nanoparticles at 1.5 Tesla. Diabetes 55, 2931–2938 (2006).
Shapiro, E.M., Sharer, K., Skrtic, S. & Koretsky, A.P. In vivo detection of single cells by MRI. Magn. Reson. Med. 55, 242–249 (2006).
Shen, F. et al. Encapsulation of recombinant cells with a novel magnetized alginate for magnetic resonance imaging. Hum. Gene Ther. 16, 971–984 (2005).
Hunkeler, D. et al. Objectively assessing bioartificial organs. Ann. NY Acad. Sci. 944, 456–471 (2001).
Cunningham, C.H. et al. Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn. Reson. Med. 53, 999–1005 (2005).
White, S.A. et al. The risks of total pancreatectomy and splenic islet autotransplantation. Cell Transplant. 9, 19–24 (2000).
Arepally, A., Karmarkar, P.V., Weiss, C. & Atalar, E. Percutaneous MR imaging-guided transvascular access of mesenteric venous system: study in swine model. Radiology 238, 113–118 (2006).
Karmarkar, P.V. et al. MR-trackable intramyocardial injection catheter. Magn. Reson. Med. 51, 1163–1172 (2004).
Lim, F. & Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 210, 908–910 (1980).
Walczak, P., Kedziorek, D.A., Gilad, A.A., Lin, S. & Bulte, J.W. Instant MR labeling of stem cells using magnetoelectroporation. Magn. Reson. Med. 54, 769–774 (2005).
Acknowledgements
A.A. Gilad and J. Ruiz-Cabello assisted with MRI, K. Schuleri with photography, and G. Clark with discussing clinical translation. Human islets were provided by the National Islet Cell Resource Center, and we thank in particular G. Weir. This work was supported by grants from the National Institutes of Health (K08 EB004348 to A.A., RO1 EB007825 to J.W.M.B., and RO1 NS045062 to J.W.M.B.). B.B. is a Howard Hughes Medical Institute Research Training Fellow and Henry Strong Denison Research Scholar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have filed a patent application relating to the work described in this paper.
Supplementary information
Supplementary Text and Figures
Supplementary Fig. 1, Supplementary Table 1, Supplementary Methods. (PDF 1741 kb)
Supplementary Movie 1
Magnetoencapsulation allows for real-time MR-guided targeted delivery. (MOV 3779 kb)
Supplementary Movie 2
Liver engraftment of MCs. (MOV 3747 kb)
Rights and permissions
About this article
Cite this article
Barnett, B., Arepally, A., Karmarkar, P. et al. Magnetic resonance–guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nat Med 13, 986–991 (2007). https://doi.org/10.1038/nm1581
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nm1581
This article is cited by
-
B-Glycine as a marker for β cell imaging and β cell mass evaluation
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Preparation and in vivo imaging of a novel potential αvβ3 targeting PET/MRI dual-modal imaging agent
Journal of Radioanalytical and Nuclear Chemistry (2022)
-
In Vivo MRI Tracking of Tumor Vaccination and Antigen Presentation by Dendritic Cells
Molecular Imaging and Biology (2022)
-
Facilitating islet transplantation using a three-step approach with mesenchymal stem cells, encapsulation, and pulsed focused ultrasound
Stem Cell Research & Therapy (2020)
-
Quantitative CT and 19F-MRI tracking of perfluorinated encapsulated mesenchymal stem cells to assess graft immunorejection
Magnetic Resonance Materials in Physics, Biology and Medicine (2019)