Abstract
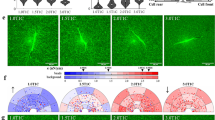
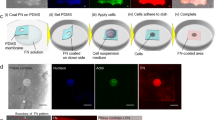
Microscale fabrication of three-dimensional (3D) extracellular matrices (ECMs) can be used to mimic the often inhomogeneous and anisotropic properties of native tissues1,2,3 and to construct in vitro cellular microenvironments4,5,6. Cellular contraction of fibrous natural ECMs (such as fibrin and collagen I) can detach matrices from their surroundings and destroy intended geometry7,8,9. Here, we demonstrate in situ collagen fibre assembly (the nucleation and growth of new collagen fibres from preformed collagen fibres at an interface) to anchor together multiple phases of cell-seeded 3D hydrogel-based matrices against cellular contractile forces. We apply this technique to stably interface multiple microfabricated 3D natural matrices (containing collagen I, Matrigel, fibrin or alginate); each phase can be seeded with cells and designed to permit cell spreading. With collagen-fibre-mediated interfacing, microfabricated 3D matrices maintain stable interfaces (the individual phases do not separate from each other) over long-term culture (at least 3 weeks) and support spatially restricted development of multicellular structures within designed patterns. The technique enables construction of well-defined and stable patterns of a variety of 3D ECMs formed by diverse mechanisms (including temperature-, ion- and enzyme-mediated crosslinking), and presents a simple approach to interface multiple 3D matrices for biological studies and tissue engineering.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mikos, A. G. et al. Engineering complex tissues. Tissue Eng. 12, 3307–3339 (2006).
Lee, C. S. et al. Integration of layered chondrocyte-seeded alginate hydrogel scaffolds. Biomaterials 28, 2987–2993 (2007).
Marenzana, M., Kelly, D. J., Prendergast, P. J. & Brown, R. A. A collagen-based interface construct for the assessment of cell-dependent mechanical integration of tissue surfaces. Cell Tissue Res. 327, 293–300 (2007).
Nelson, C. M. & Tien, J. Microstructured extracellular matrices in tissue engineering and development. Curr. Opin. Biotechnol. 17, 518–523 (2006).
Nelson, C. M., Vanduijn, M. M., Inman, J. L., Fletcher, D. A. & Bissell, M. J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314, 298–300 (2006).
Khademhosseini, A., Langer, R., Borenstein, J. & Vacanti, J. P. Microscale technologies for tissue engineering and biology. Proc. Natl Acad. Sci. USA 103, 2480–2487 (2006).
Rowe, S. L. & Stegemann, J. P. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules 7, 2942–2948 (2006).
Sieminski, A. L., Hebbel, R. P. & Gooch, K. J. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp. Cell Res. 297, 574–584 (2004).
Meshel, A. S., Wei, Q., Adelstein, R. S. & Sheetz, M. P. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nature Cell Biol. 7, 157–164 (2005).
Davis, G. E., Bayless, K. J. & Mavila, A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat. Rec. 268, 252–275 (2002).
Isenberg, B. C., Williams, C. & Tranquillo, R. T. Endothelialization and flow conditioning of fibrin-based media-equivalents. Ann. Biomed. Eng. 34, 971–985 (2006).
Radisic, M. et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl Acad. Sci. USA 101, 18129–18134 (2004).
Golden, A. P. & Tien, J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip 7, 720–725 (2007).
Ling, Y. et al. A cell-laden microfluidic hydrogel. Lab Chip 7, 756–762 (2007).
Tan, W. & Desai, T. A. Microscale multilayer cocultures for biomimetic blood vessels. J. Biomed. Mater. Res. A 72, 146–160 (2005).
Albrecht, D. R., Underhill, G. H., Wassermann, T. B., Sah, R. L. & Bhatia, S. N. Probing the role of multicellular organization in three-dimensional microenvironments. Nature Methods 3, 369–375 (2006).
Paguirigan, A. & Beebe, D. J. Gelatin based microfluidic devices for cell culture. Lab Chip 6, 407–413 (2006).
Cheung, Y. K., Gillette, B. M., Zhong, M., Ramcharan, S. & Sia, S. K. Direct patterning of composite biocompatible microstructures using microfluidics. Lab Chip 7, 574–579 (2007).
Albrecht, D. R., Underhill, G. H., Mendelson, A. & Bhatia, S. N. Multiphase electropatterning of cells and biomaterials. Lab Chip 7, 702–709 (2007).
Choi, N. W. et al. Microfluidic scaffolds for tissue engineering. Nature Mater. 6, 908–915 (2007).
Cabodi, M. et al. A microfluidic biomaterial. J. Am. Chem. Soc. 127, 13788–13789 (2005).
Lee, P., Lin, R., Moon, J. & Lee, L. P. Microfluidic alignment of collagen fibers for in vitro cell culture. Biomed. Microdevices 8, 35–41 (2006).
Brightman, A. O. et al. Time-lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers 54, 222–234 (2000).
Helm, C. L., Zisch, A. & Swartz, M. A. Engineered blood and lymphatic capillaries in 3-D VEGF-fibrin-collagen matrices with interstitial flow. Biotechnol. Bioeng. 96, 167–176 (2007).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Boontheekul, T., Kong, H. J. & Mooney, D. J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 26, 2455–2465 (2005).
Kamei, M. et al. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442, 453–456 (2006).
Campbell, B. H., Clark, W. W. & Wang, J. H. A multi-station culture force monitor system to study cellular contractility. J. Biomech. 36, 137–140 (2003).
Freyman, T. M., Yannas, I. V., Yokoo, R. & Gibson, L. J. Fibroblast contraction of a collagen-GAG matrix. Biomaterials 22, 2883–2891 (2001).
Masuda, K., Sah, R. L., Hejna, M. J. & Thonar, E. J. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: The alginate-recovered-chondrocyte (ARC) method. J. Orthop. Res. 21, 139–148 (2003).
Weinand, C. et al. Comparison of hydrogels in the in vivo formation of tissue-engineered bone using mesenchymal stem cells and beta-tricalcium phosphate. Tissue Eng. 13, 757–765 (2007).
Acknowledgements
We acknowledge a Scientist Development Grant from the American Heart Association and an NSF CAREER award.
Author information
Authors and Affiliations
Contributions
B.M.G. and S.K.S. conceived the project, designed the experiments, analysed the data, developed the interpretations and wrote the manuscript. B.M.G. carried out the bulk of the experiments. J.A.J., B.T., G.J.Y., A.B. and M.Z. provided suggestions for developing the method and carried out the remainder of the experiments under the guidance of B.M.G. and S.K.S. All authors have agreed to the content of the manuscript, including the data as presented.
Corresponding author
Supplementary information
Supplementary Information
Supplementary Figures S1–S6 (PDF 2345 kb)
Rights and permissions
About this article
Cite this article
Gillette, B., Jensen, J., Tang, B. et al. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nature Mater 7, 636–640 (2008). https://doi.org/10.1038/nmat2203
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nmat2203
This article is cited by
-
Enhanced osteogenesis of quasi-three-dimensional hierarchical topography
Journal of Nanobiotechnology (2019)
-
Formation of Multi-Component Extracellular Matrix Protein Fibers
Scientific Reports (2018)
-
Nonlinear mechanics of hybrid polymer networks that mimic the complex mechanical environment of cells
Nature Communications (2017)
-
Templated Assembly of Collagen Fibers Directs Cell Growth in 2D and 3D
Scientific Reports (2017)
-
An implantable compound-releasing capsule triggered on demand by ultrasound
Scientific Reports (2016)