Abstract
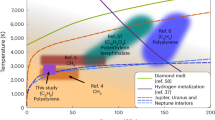
Since Ross proposed that there might be ‘diamonds in the sky’ in 1981 (ref. 1), the idea of significant quantities of pure carbon existing in giant planets such as Uranus and Neptune has gained both experimental2 and theoretical3 support. It is now accepted that the high-pressure, high-temperature behaviour of carbon is essential to predicting the evolution and structure of such planets4. Still, one of the most defining of thermal properties for diamond, the melting temperature, has never been directly measured. This is perhaps understandable, given that diamond is thermodynamically unstable, converting to graphite before melting at ambient pressure, and tightly bonded, being the strongest bulk material known5,6. Shock-compression experiments on diamond reported here reveal the melting temperature of carbon at pressures of 0.6–1.1 TPa (6–11 Mbar), and show that crystalline diamond can be stable deep inside giant planets such as Uranus and Neptune1,2,3,4,7. The data indicate that diamond melts to a denser, metallic fluid—with the melting curve showing a negative Clapeyron slope—between 0.60 and 1.05 TPa, in good agreement with predictions of first-principles calculations8. Temperature data at still higher pressures suggest diamond melts to a complex fluid state, which dissociates at shock pressures between 1.1 and 2.5 TPa (11–25 Mbar) as the temperatures increase above 50,000 K.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ross, M. The ice layer in Uranus and Neptune—diamonds in the sky? Nature 292, 435–436 (1981).
Benedetti, L. R. et al. Dissociation of CH4 at high pressures and temperatures: Diamond formation in giant planet interiors? Science 286, 100–102 (1999).
Ancilotto, F., Chiarotti, G. L., Scandolo, S. & Tosatti, E. Dissociation of methane into hydrocarbons at extreme (planetary) pressure and temperature. Science 275, 1288–1290 (1997).
Guillot, T. The interiors of giant planets: Models and outstanding questions. Annu. Rev. Earth Planet. Sci. 33, 493–530 (2005).
Bundy, F. P. Melting of graphite at very high pressure. J. Chem. Phys. 38, 618–630 (1963).
Togaya, M. in Science and Technology of New Diamond (eds Saitro, S., Fukunaga, O. & Yoshikawa, M.) 369–373 (KTK Scientific Publishers, 1990).
Bradley, D. K. et al. Shock compressing diamond to a conducting fluid. Phys. Rev. Lett. 93, 195506 (2004).
Correa, A. A., Bonev, S. A. & Galli, G. Carbon under extreme conditions: Phase boundaries and electronic properties from first-principles theory. Proc. Natl Acad. Sci. USA 103, 1204–1208 (2006).
Grumbach, M. P. & Martin, R. M. Phase diagram of carbon at high pressures and temperatures. Phys. Rev. B 54, 15730–15741 (1996).
Wu, C. J., Glosli, J. N., Galli, G. & Ree, F. H. Liquid–liquid phase transition in elemental carbon: A first-principles investigation. Phys. Rev. Lett. 89, 135701 (2002).
Wang, X.-F., Scandolo, S. & Car, R. Carbon phase diagram from ab initio molecular dynamics. Phys. Rev. Lett. 95, 185701 (2005).
Weathers, M. S. & Bassett, W. A. Melting of carbon at 50–300 kbar. Phys. Chem. Minerals 15, 105–112 (1987).
Bundy, F. P. Direct conversion of graphite to diamond in static pressure apparatus. J. Chem. Phys. 38, 631–643 (1963).
Gust, W. H. Phase transition and shock-compression parameters to 120 GPa for three types of graphite and for amorphous carbon. Phys. Rev. B 22, 4744–4756 (1980).
Fried, L. E. & Howard, W. M. Explicit Gibbs free energy equation of state applied to the carbon phase diagram. Phys. Rev. B 61, 8734–8743 (2000).
Shaner, F. W., Brown, J. M., Swenson, C. A. & McQueen, R. G. Sound velocity of carbon at high pressures. J. Phys. (Paris), Colloq. 45, C8-235–237 (1984).
Hicks, D. G. et al. High precision measurements of the diamond Hugoniot in and above the melt region. Phys. Rev. B 78, 174102 (2008).
Nagao, H. et al. Hugoniot measurement of diamond under laser shock compression up to 2 TPa. Phys. Plasmas 13, 052705 (2006).
Knudson, M. D., Desjarlais, M. P. & Dolan, D. H. Shock-wave exploration of the high-pressure phases of carbon. Science 322, 1822–1825 (2008).
Brygoo, S. et al. Laser-shock compression of diamond and evidence of a negative slope melting curve. Nature Mater. 6, 274–277 (2007).
Hicks, D. G. et al. Dissociation of liquid silica at high pressures and temperatures. Phys. Rev. Lett. 97, 025502 (2006).
Lyon, S. P. & Johnson, J. D. Diamond Sesame Table. Report No. LA-UR-92-3407 (Los Alamos National Laboratory, 1992).
Miller, J. E. et al. Streaked optical pyrometer system for laser-driven shock-wave experiments on OMEGA. Rev. Sci. Instrum. 78, 034903 (2006).
Collins, G. W. et al. Temperature measurements of shock compressed liquid deuterium up to 230 GPa. Phys. Rev. Lett. 87, 165504 (2001).
Kormer, S. B. Optical study of the characteristics of shock-compressed condensed dielectrics. Sov. Phys. Usp. 11, 229–254 (1968).
Scandolo, S., Chiarotti, G. L. & Tosatti, E. SC4: A metallic phase of carbon at terapascal pressures. Phys. Rev. B 53, 5051–5054 (1996).
Keeler, R. N. & Royce, E. B. in Physics of High Energy, Density (eds Caldirola, P. & Knoepfel, H.) 88 (Academic, 1971).
Goodstein, D. L. States of Matter (Dover, 1985).
Hubbard, W. B. et al. Interior structure of Neptune: Comparison with Uranus. Science 253, 648–651 (1991).
Stanley, S. & Bloxham, J. Convective-region geometry as the cause of Uranus’ and Neptune’s unusual magnetic fields. Nature 428, 151–153 (2004).
Acknowledgements
We thank W. Unites for sample support. This work was carried out under the auspices of the US Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
Author information
Authors and Affiliations
Contributions
J.H.E., D.G.H., P.M.C., D.K.B. and T.R.B. designed and carried out the experiments. J.H.E., D.G.H., P.M.C. and R.S.M. carried out the analysis; J.E.M. and T.R.B. calibrated the SOP. R.J. and G.W.C. administered the experiment and J.H.E., R.J. and G.W.C. wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 673 kb)
Rights and permissions
About this article
Cite this article
Eggert, J., Hicks, D., Celliers, P. et al. Melting temperature of diamond at ultrahigh pressure. Nature Phys 6, 40–43 (2010). https://doi.org/10.1038/nphys1438
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nphys1438
This article is cited by
-
Melting curve of superionic ammonia at planetary interior conditions
Nature Physics (2023)
-
Materials under extreme conditions using large X-ray facilities
Nature Reviews Methods Primers (2023)
-
Non-thermal evolution of dense plasmas driven by intense x-ray fields
Communications Physics (2023)
-
Metastability of diamond ramp-compressed to 2 terapascals
Nature (2021)
-
Evidence of hydrogen−helium immiscibility at Jupiter-interior conditions
Nature (2021)