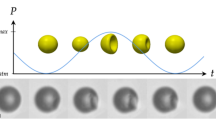
Abstract
In fluids, pressure-driven cavitation bubbles have a nonlinear response that can lead to extremely high core-energy densities during the collapse phase—a process underpinning phenomena such as sonoluminescence1 and plasma formation2. If cavitation occurs near a rigid surface, the bubbles tend to collapse asymmetrically, often forming fast-moving liquid jets that may create localized surface damage3. As encapsulated microbubbles are commonly used to improve echo generation in diagnostic ultrasound imaging, it is possible that such cavitation could also lead to jet-induced tissue damage. Certainly ultrasonic irradiation (insonation) of cells in the presence of microbubbles can lead to enhanced membrane permeabilization and molecular uptake (sonoporation)4,5,6,7, but, although the mechanism during low-intensity insonation is clear8, experimental corroboration for higher pressure regimes has remained elusive. Here we show direct observational evidence that illuminates the energetic micrometre-scale interactions between individual cells and violently cavitating shelled microbubbles. Our data suggest that sonoporation at higher intensities may arise through a synergistic interplay involving several distinct processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hilgenfeldt, S., Grossmann, S. & Lohse, D. A simple explanation of light emission in sonoluminescence. Nature 398, 402–405 (1999).
Flannigan, D. J. & Suslick, K. S. Plasma formation and temperature measurement during single bubble cavitation. Nature 434, 52–55 (2005).
Benjamin, T. B. & Ellis, A. T. The collapse of cavitation bubbles and the pressure thereby produced against solid boundaries. Phil. Trans. R. Soc. Lond. A 260, 221–245 (1966).
Ward, M., Wu, J. & Chiu, J. F. Ultrasound induced cell lysis and sonoporation enhanced by contrast agents. J. Acoust. Soc. Am. 105, 2951–2957 (1999).
Feril, L. B. et al. Enhancement of ultrasound-induced apoptosis and cell lysis by echo contrast agents. Ultrasound Med. Biol. 29, 331–337 (2003).
Honda, H., Kondo, T., Zhao, Q. L., Feril, L. B. & Kitagawa, H. Role of intracellular calcium ions and reactive oxygen species in apoptosis induced by ultrasound. Ultrasound Med. Biol. 30, 683–692 (2004).
Miller, D. L. & Song, J. Tumour growth reduction and DNA transfer by cavitation-enhanced high intensity focused ultrasound in vivo. Ultrasound Med. Biol. 29, 887–893 (2003).
Marmottant, P. & Hilgenfeldt, S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 423, 153–156 (2003).
Canatella, P. J. & Prausnitz, M. R. Prediction and optimization of gene transfection and drug delivery by electroporation. Gene Therapy 8, 1464–1469 (2001).
Tirlapur, U. K. & König, K. Targeted transfection by femtosecond laser. Nature 418, 290–291 (2002).
Gahagan, K. T. & Swartzlander, G. A. Trapping of low-index microparticles in an optical vortex. J. Opt. Soc. Am. B 15, 524–534 (1998).
Phillip, A. & Lauterborn, W. Cavitation erosion by single laser-produced bubbles. J. Fluid Mech. 361, 75–116 (1998).
Plesset, M. S. & Chapman, R. B. Collapse of an initially spherical vapour cavity in the neighbourhood of a solid boundary. J. Fluid. Mech. 47, 283–290 (1971).
Ohl, C. D. & Ikink, R. Shock wave induced jetting of micron size bubbles. Phys. Rev. Lett. 90, 214502 (2003).
Van Wamel, A., Bouakaz, A., Versluis, M. & de Jong, N. Micromanipulation of endothelial cells: Ultrasound-microbubble-cell interactions. Ultrasound Med. Biol. 30, 1255–1258 (2004).
Wolfram, B., Mettin, R., Kurz, T. & Lauterborn, W. Observations of pressure wave excited contrast agent microbubbles in the vicinity of cells. Appl. Phys. Lett. 81, 5060–5062 (2002).
Postema, M., Van Wamel, A., Lancée, C. T. & de Jong, N. Ultrasound-induced encapsulated microbubble phenomena. Ultrasound Med. Biol. 30, 827–840 (2004).
Postema, M. Medical Bubbles (Universal, Veenedaal, Netherlands, 2004).
Weaver, J. C. & Chizmadzev, Y. A. Theory of electroporation: A review. Bioelectrochem. Bioenerget. 41, 135–160 (1996).
Steinhardt, R. A., Bi, G. & Alderton, J. M. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science 263, 390–393 (1994).
Deng, C. X., Sieling, F., Pan, H. & Cui, J. Ultrasound induced cell membrane porosity. Ultrasound Med. Biol. 30, 519–526 (2004).
Guzman, H. R., Nguyen, D. X., Khan, S. & Prausnitz, M. R. Ultrasound-mediated disruption of cell membranes. II. Heterogeneous effects on cells. J. Acoust. Soc. Am. 110, 597–606 (2001).
Boal, D. Mechanics of the Cell (Cambridge Univ. Press, Cambridge, 2002).
Rotch, C., Jacobson, K. & Radmacher, M. Dimensional and mechanical dynamics of active and stable edges in motile fibroblasts investigated using atomic force microscopy. Proc. Natl Acad. Sci. USA 96, 921–926 (1999).
Brujan, E. A. The role of cavitation microjets in the therapeutic applications of ultrasound. Ultrasound Med. Biol. 30, 381–387 (2004).
Acknowledgements
We thank the EPSRC, SHEFC and National Institutes for Health for financial support and the EPSRC equipment loan pool (A. Walker and P. Anthony) for use of the high-speed imaging systems. We also gratefully acknowledge advice and assistance from V. Zarnitzyn and M. Postema (sonoporation), D. McLean (technical construction), M. McDonald (optical trapping) and J. Christophe Bourdon and P. Robertson (cell culture).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M. R. P. is on the advisory board of Cytodome. The rest of the authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Prentice, P., Cuschieri, A., Dholakia, K. et al. Membrane disruption by optically controlled microbubble cavitation. Nature Phys 1, 107–110 (2005). https://doi.org/10.1038/nphys148
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nphys148
This article is cited by
-
Novel opto-fluidic drug delivery system for efficient cellular transfection
Journal of Nanobiotechnology (2023)
-
Use of stimulatory responsive soft nanoparticles for intracellular drug delivery
Nano Research (2023)
-
The effect of scalable PDMS gas-entrapping microstructures on the dynamics of a single cavitation bubble
Scientific Reports (2022)
-
Deep laser microscopy using optical clearing by ultrasound-induced gas bubbles
Nature Photonics (2022)
-
Low-intensity ultrasound inhibits melanoma cell proliferation in vitro and tumor growth in vivo
Journal of Medical Ultrasonics (2021)