Abstract
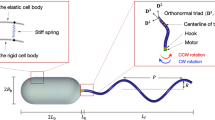
The bacterium Caulobacter crescentus swims by rotating a single right-handed helical filament. These cells have two swimming modes: a pusher mode, in which clockwise (CW) rotation of the filament thrusts the cell body forwards1, and a puller mode, in which counterclockwise (CCW) rotation pulls it backwards2. The situation is reversed in Escherichia coli, a bacterium that rotates several left-handed filaments CCW to drive the cell body forwards. The flagellar motor in E. coli generates more torque in the CCW direction than the CW direction in swimming cells3,4. However, C. crescentus and other bacteria with single filaments swim forwards and backwards at similar speeds, prompting the assumption that motor torques in the two modes are the same5,6. Here, we present evidence that motors in C. crescentus develop higher torques in the puller mode than in the pusher mode, and suggest that the anisotropy in torque generation is similar in the two species, despite the differences in filament handedness and motor bias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Koyasu, S. & Shirakihara, Y. Caulobacter crescentus flagellar filament has a right-handed helical form. J. Mol. Biol. 173, 125–130 (1984).
Lauga, E. & Powers, T. R. The hydrodynamics of swimming microorganisms. Rep. Prog. Phys. 72, 096601 (2009).
Chen, X. B. & Berg, H. C. Torque-speed relationship of the flagellar rotary motor of Escherichia coli. Biophys. J. 78, 1036–1041 (2000).
Yuan, J., Fahrner, K. A., Turner, L. & Berg, H. C. Asymmetry in the clockwise and counterclockwise rotation of the bacterial flagellar motor. Proc. Natl Acad. Sci. USA 107, 12846–12849 (2010).
Li, G. & Tang, J. X. Low flagellar motor torque and high swimming efficiency of Caulobacter crescentus swarmer cells. Biophys. J. 91, 2726–2734 (2006).
Liu, B. et al. Helical motion of the cell body enhances Caulobacter crescentus motility. Proc. Natl Acad. Sci. USA 111, 11252–11256 (2014).
Rafai, S., Jibuti, L. & Peyla, P. Effective viscosity of microswimmer suspensions. Phys. Rev. Lett. 104, 098102 (2010).
Saintillan, D. The dilute rheology of swimming suspensions: A simple kinetic model. Exp. Mech. 50, 1275–1281 (2010).
Underhill, P. T., Hernandez-Ortiz, J. P. & Graham, M. D. Diffusion and spatial correlations in suspensions of swimming particles. Phys. Rev. Lett. 100, 248101 (2008).
Watari, N. & Larson, R. G. The hydrodynamics of a run-and-tumble bacterium propelled by polymorphic helical flagella. Biophys. J. 98, 12–17 (2010).
Hatwalne, Y., Ramaswamy, S., Rao, M. & Simha, R. A. Rheology of active-particle suspensions. Phys. Rev. Lett. 92, 118101 (2004).
Magariyama, Y. et al. Difference in bacterial motion between forward and backward swimming caused by the wall effect. Biophys. J. 88, 3648–3658 (2005).
Goto, T., Nakata, K., Baba, K., Nishimura, M. & Magariyama, Y. A fluid-dynamic interpretation of the asymmetric motion of singly flagellated bacteria swimming close to a boundary. Biophys. J. 89, 3771–3779 (2005).
Theves, M., Taktikos, J., Zaburdaev, V., Stark, H. & Beta, C. A bacterial swimmer with two alternating speeds of propagation. Biophys. J. 105, 1915–1924 (2013).
Lele, P. P., Hosu, B. G. & Berg, H. C. Dynamics of mechanosensing in the bacterial flagellar motor. Proc. Natl Acad. Sci. USA 110, 11839–11844 (2013).
Ausmees, N., Kuhn, J. R. & Jacobs-Wagner, C. The bacterial cytoskeleton: An intermediate filament-like function in cell shape. Cell 115, 705–713 (2003).
Seltmann, G. & Holst, O. The Bacterial Cell Wall (Springer, 2001).
Lauga, E., DiLuzio, W. R., Whitesides, G. M. & Stone, H. A. Swimming in circles: Motion of bacteria near solid boundaries. Biophys. J. 90, 400–412 (2006).
Rodenborn, B., Chen, C. H., Swinney, H. L., Liu, B. & Zhang, H. P. Propulsion of microorganisms by a helical flagellum. Proc. Natl Acad. Sci. USA 110, E338–E347 (2013).
Lighthill, J. Flagellar hydrodynamics–Neumann, JV Lecture, 1975. Soc. Ind. Appl. Math. Rev. 18, 161–230 (1976).
Stallmeyer, M. J., Hahnenberger, K. M., Sosinsky, G. E., Shapiro, L. & DeRosier, D. J. Image reconstruction of the flagellar basal body of Caulobacter crescentus. J. Mol. Biol. 205, 511–518 (1989).
Faulds-Pain, A. et al. Flagellin redundancy in Caulobacter crescentus and its implications for flagellar filament assembly. J. Bacteriol. 193, 2695–2707 (2011).
Shapiro, L. Differentiation in the Caulobacter cell cycle. Annu. Rev. Microbiol. 30, 377–407 (1976).
Thanbichler, M., Iniesta, A. A. & Shapiro, L. A comprehensive set of plasmids for vanillate- and xylose-inducible gene expression in Caulobacter crescentus. Nucl. Acids Res. 35, e137 (2007).
Crocker, J. C. & Grier, D. G. Methods of digital video microscopy for colloidal studies. J. Colloid Interf. Sci. 179, 298–310 (1996).
Lele, P. P., Branch, R. W., Nathan, V. S. & Berg, H. C. Mechanism for adaptive remodeling of the bacterial flagellar switch. Proc. Natl Acad. Sci. USA 109, 20018–20022 (2012).
Acknowledgements
We thank P. Aldridge, C. Jacobs-Wagner and M. Laub for strains. We are grateful to I. Hug and U. Jenal for strains, reagents and advice. The work was supported by National Institutes of Health Grant AI016478.
Author information
Authors and Affiliations
Contributions
P.P.L. and H.C.B. designed the work; P.P.L., T.R., A.S. and Y.C. performed the research; P.P.L., T.R. and Y.C. analysed the data; P.P.L., T.R. and H.C.B. developed the experimental set-up; and P.P.L. and H.C.B. wrote the paper with inputs from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1144 kb)
Supplementary Movie 1
Supplementary Movie (AVI 1778 kb)
Supplementary Movie 2
Supplementary Movie (AVI 2463 kb)
Supplementary Movie 3
Supplementary Movie (AVI 536 kb)
Supplementary Movie 4
Supplementary Movie (AVI 660 kb)
Supplementary Movie 5
Supplementary Movie (AVI 1234 kb)
Rights and permissions
About this article
Cite this article
Lele, P., Roland, T., Shrivastava, A. et al. The flagellar motor of Caulobacter crescentus generates more torque when a cell swims backwards. Nature Phys 12, 175–178 (2016). https://doi.org/10.1038/nphys3528
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nphys3528
This article is cited by
-
Linear motor driven-rotary motion of a membrane-permeabilized ghost in Mycoplasma mobile
Scientific Reports (2018)
-
Torque, but not FliL, regulates mechanosensitive flagellar motor-function
Scientific Reports (2017)
-
Experimental characterization of helical swimming trajectories in circular channels
Microfluidics and Nanofluidics (2017)