Abstract
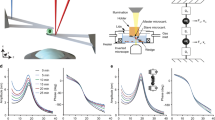
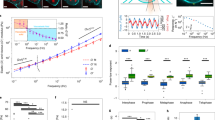
Living cells are viscoelastic materials, dominated by an elastic response on timescales longer than a millisecond1. On shorter timescales, the dynamics of individual cytoskeleton filaments are expected to emerge, but active microrheology measurements on cells accessing this regime are scarce2. Here, we develop high-frequency microrheology experiments to probe the viscoelastic response of living cells from 1 Hz to 100 kHz. We report the viscoelasticity of different cell types under cytoskeletal drug treatments. On previously inaccessible short timescales, cells exhibit rich viscoelastic responses that depend on the state of the cytoskeleton. Benign and malignant cancer cells revealed remarkably different scaling laws at high frequencies, providing a unique mechanical fingerprint. Microrheology over a wide dynamic range—up to the frequency characterizing the molecular components—provides a mechanistic understanding of cell mechanics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fabry, B. et al. Scaling the microrheology of living cells. Phys. Rev. Lett. 87, 148102–148105 (2001).
Deng, L. H. et al. Fast and slow dynamics of the cytoskeleton. Nat. Mater. 5, 636–640 (2006).
Petersen, N. O., McConnaughey, W. B. & Elson, E. L. Dependence of locally measured cellular deformability on position on the cell, temperature, and cytochalasin B. Proc. Natl Acad. Sci. USA 79, 5327–5331 (1982).
Kollmannsberger, P. & Fabry, B. Linear and nonlinear rheology of living cells. Annu. Rev. Mater. Res. 41, 75–97 (2011).
Bausch, A. & Kroy, K. A bottom-up approach to cell mechanics. Nat. Phys. 2, 231–238 (2006).
Alcaraz, J. et al. Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys. J. 84, 2071–2079 (2003).
Broedersz, C. P. & MacKintosh, F. C. Modeling semiflexible polymer networks. Rev. Mod. Phys. 86, 995–1036 (2014).
Gittes, F. et al. Microscopic viscoelasticity: shear moduli of soft materials determined from thermal fluctuations. Phys. Rev. Lett. 79, 3286–3289 (1997).
Amblard, F. et al. Subdiffusion and anomalous local viscoelasticity in actin networks. Phys. Rev. Lett. 77, 4470–4473 (1996).
Isambert, H. & Maggs, A. Dynamics and rheology of actin solutions. Macromolecules 29, 1036–1040 (1996).
Caspi, A., Elbaum, M., Granek, R., Lachish, A. & Zbaida, D. Semiflexible polymer network: a view from inside. Phys. Rev. Lett. 80, 1106–1109 (1998).
Koenderink, G. H., Atakhorrami, M., MacKintosh, F. C. & Schmidt, C. F. High-frequency stress relaxation in semiflexible polymer solutions and networks. Phys. Rev. Lett. 96, 138307 (2006).
Semmrich, C. Glass transition and rheological redundancy in F-actin solutions. Proc. Natl Acad. Sci. USA 104, 20199–20203 (2007).
Everaers, R., Jülicher, F., Ajdari, A. & Maggs, A. C. Dynamic fluctuations of semiflexible filaments. Phys. Rev. Lett. 82, 3717–3720 (1999).
Mizuno, D., Tardin, C., Schmidt, C. F. & MacKintosh, F. C. Nonequilibrium mechanics of active cytoskeletal networks. Science 315, 370–373 (2007).
Hoffman, B. D., Massiera, G., Van Citters, K. M. & Crocker, J. C. The consensus mechanics of cultured mammalian cells. Proc. Natl Acad. Sci. USA 103, 10259–10264 (2006).
Yamada, S., Wirtz, D. & Kuo, S. C. Mechanics of living cells measured by laser tracking microrheology. Biophys. J. 78, 1736–1747 (2000).
Mahaffy, R. E., Shih, C. K., MacKintosh, F. C. & Kas, J. Scanning probe-based frequency-dependent microrheology of polymer gels and biological cells. Phys. Rev. Lett. 85, 880–883 (2000).
Cartagena-Rivera, A. X., Wang, W.-H., Geahlen, R.L. & Raman, A. Fast, multi-frequency, and quantitative nanomechanical mapping of live cells using the atomic force microscope. Sci. Rep. 5, 11692 (2015).
Gavara, N. & Chadwick, R. S. Noncontact microrheology at acoustic frequencies using frequency-modulated atomic force microscopy. Nat. Methods 7, 650–654 (2010).
Ando, T. et al. A high-speed atomic force microscope for studying biological macromolecules. Proc. Natl Acad. Sci. USA 98, 12468–12472 (2001).
Rico, F., Gonzalez, L., Casuso, I., Puig-Vidal, M. & Scheuring, S. High-speed force spectroscopy unfolds titin at the velocity of molecular dynamics simulations. Science 342, 741–743 (2013).
Alcaraz, J. et al. Correction of microrheological measurements of soft samples with atomic force microscopy for the hydrodynamic drag on the cantilever. Langmuir 18, 716–721 (2002).
Janovjak, H. J., Struckmeier, J. & Muller, D. J. Hydrodynamic effects in fast AFM single-molecule force measurements. Eur. Biophys. J. Biophys. Lett. 34, 91–96 (2005).
Schnurr, B., Gittes, F., MacKintosh, F. & Schmidt, C. Determining microscopic viscoelasticity in flexible and semiflexible polymer networks from thermal fluctuations. Macromolecules 30, 7781–7792 (1997).
Colom, A., Casuso, I., Rico, F. & Scheuring, S. A hybrid high-speed atomic force–optical microscope for visualizing single membrane proteins on eukaryotic cells. Nat. Commun. 4, 2155 (2013).
Gavara, N. & Chadwick, R. S. Determination of the elastic moduli of thin samples and adherent cells using conical atomic force microscope tips. Nat. Nanotech. 7, 733–736 (2012).
Takahashi, R. & Okajima, T. Mapping power-law rheology of living cells using multi-frequency force modulation atomic force microscopy. Appl. Phys. Lett. 107, 173702 (2015).
Rigato, A., Rico, F., Eghiaian, F., Piel, M. & Scheuring, S. Atomic force microscopy mechanical mapping of micropatterned cells shows adhesion geometry-dependent mechanical response on local and global scales. ACS Nano 9, 5846–5856 (2015).
Clark, A. G., Dierkes, K. & Paluch, E. K. Monitoring actin cortex thickness in live cells. Biophys. J. 105, 570–580 (2013).
Zhou, E., Quek, S. & Lim, C. Power-law rheology analysis of cells undergoing micropipette aspiration. Biomech. Model. Mechanobiol. 9, 563–572 (2010).
Fabry, B. et al. Time scale and other invariants of integrative mechanical behavior in living cells. Phys. Rev. E 68, 041914 (2003).
Obermayer, B. & Frey, E. Tension dynamics and viscoelasticity of extensible wormlike chains. Phys. Rev. E 80, 040801 (2009).
Eghiaian, F., Rigato, A. & Scheuring, S. Structural, mechanical, and dynamical variability of the actin cortex in living cells. Biophys. J. 108, 1330–1340 (2015).
Guck, J. et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 88, 3689–3698 (2005).
Guo, M. et al. Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell 158, 822–832 (2014).
Bertseva, E. Optical trapping microrheology in cultured human cells. Eur. Phys. J. E 35, 1–8 (2012).
Kroy, K. & Glaser, J. The glassy wormlike chain. New J. Phys. 9, 416 (2007).
Ahmed, W. W. et al. Active mechanics reveal molecular-scale force kinetics in living oocytes. Preprint at http://arXiv.org/abs/151008299 (2015).
Bursac, P. et al. Cytoskeletal remodelling and slow dynamics in the living cell. Nat. Mater. 4, 557–561 (2005).
Turlier, H. et al. Equilibrium physics breakdown reveals the active nature of red blood cell flickering. Nat. Phys. 12, 513–519 (2016).
Higgins, M. J. et al. Noninvasive determination of optical lever sensitivity in atomic force microscopy. Rev. Sci. Instrum. 77, 013701 (2006).
Sader, J. E., Chon, J. W. M. & Mulvaney, P. Calibration of rectangular atomic force microscope cantilevers. Rev. Sci. Instrum. 70, 3967–3969 (1999).
Hutter, J. L. Comment on tilt of atomic force microscope cantilevers: effect on spring constant and adhesion measurements. Langmuir 21, 2630–2632 (2005).
Rico, F. et al. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys. Rev. E. Stat. Nonlin. Soft Matter Phys. 72, 021914 (2005).
Press, W. H. Numerical Recipes 3rd Edition: The Art of Scientific Computing (Cambridge Univ. Press, 2007).
Acknowledgements
The authors thank A. Sergé, M. Lopez and N. Dusetti for generously providing cell lines and for their technical support, L. Borge from the PCC TPR2-Luminy for technical assistance and F. Eghiaian for helpful discussions. This work was supported by Agence National de la Recherche grants BioHSFS ANR-15-CE11-0007, ANR-11-LABX-0054 (Labex INFORM), ANR-11-IDEX-0001-02 (A ∗ MIDEX) and a European Research Council (ERC) Grant #310080 (MEM-STRUCT-AFM).
Author information
Authors and Affiliations
Contributions
A.R. designed and performed the experiments, analysed the data and wrote the manuscript. F.R. designed the experiments, analysed the data and wrote the manuscript. A.M. modified the high-speed-AFM scanner and helped with calibration and experiments. S.S. contributed to designing the experiments and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 583 kb)
Rights and permissions
About this article
Cite this article
Rigato, A., Miyagi, A., Scheuring, S. et al. High-frequency microrheology reveals cytoskeleton dynamics in living cells. Nature Phys 13, 771–775 (2017). https://doi.org/10.1038/nphys4104
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nphys4104
This article is cited by
-
Mechanobiology across timescales
Nature Reviews Physics (2025)
-
Cancer cells impact the microrheology of endothelial cells during physical contact or through paracrine signalling
Scientific Reports (2025)
-
Hierarchical F-actin microstructures and multi-passage viscoelasticity evolution in living cancer cells under varying glucose environment
Acta Mechanica Sinica (2025)
-
Length and time scales bridge poroelasticity and tissue architecture
Journal of Biosciences (2025)
-
Mechanical stimulation and electrophysiological monitoring at subcellular resolution reveals differential mechanosensation of neurons within networks
Nature Nanotechnology (2024)