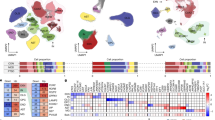
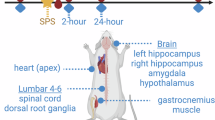
Abstract
Severe stress or trauma can cause permanent changes in brain circuitry, leading to dysregulation of fear responses and the development of posttraumatic stress disorder (PTSD). To date, little is known about the molecular mechanisms underlying stress-induced long-term plasticity in fear circuits. We addressed this question by using global gene expression profiling in an animal model of PTSD, stress-enhanced fear learning (SEFL). A total of 15 footshocks were used to induce SEFL and the volatile anesthetic isoflurane was used to suppress the behavioral effects of stress. Gene expression in lateral/basolateral amygdala was measured using microarrays at 3 weeks after the exposure to different combinations of shock and isoflurane. Shock produced robust effects on amygdalar transcriptome and isoflurane blocked or reversed many of the stress-induced changes. We used a modular approach to molecular profiles of shock and isoflurane and built a network of regulated genes, functional categories, and cell types that represent a mechanistic foundation of perturbation-induced plasticity in the amygdala. This analysis partitioned perturbation-induced changes in gene expression into neuron- and astrocyte-specific changes, highlighting a previously underappreciated role of astroglia in amygdalar plasticity. Many neuron-enriched genes were highly correlated with astrocyte-enriched genes, suggesting coordinated transcriptional responses to environmental challenges in these cell types. Several individual genes were validated using RT-PCR and behavioral pharmacology. This study is the first to propose specific cellular and molecular mechanisms underlying SEFL, an animal model of PTSD, and to nominate novel molecular and cellular targets with potential for therapeutic intervention in PTSD, including glycine and neuropeptide systems, chromatin remodeling, and gliotransmission.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abel T, Zukin RS (2008). Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol 8: 57–64.
Amat J, Matus-Amat P, Watkins LR, Maier SF (1998). Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res 812: 113–120.
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, IV edn. American Psychiatric Press: Washington, DC.
Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV et al (2007). Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience 146: 1495–1503.
Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS et al (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28: 264–278.
Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED et al (2009). 5-Hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry 67: 339–345.
Chwang WB, Arthur JS, Schumacher A, Sweatt JD (2007). The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci 27: 12732–12742.
Coufal NG, Garcia-Perez JL, Peng GE, Yeo GW, Mu Y, Lovci MT et al (2009). L1 retrotransposition in human neural progenitor cells. Nature 460: 1127–1131.
De Keyser J, Zeinstra E, Wilczak N (2004). Astrocytic beta2-adrenergic receptors and multiple sclerosis. Neurobiol Dis 15: 331–339.
Dennis KE, Levitt P (2005). Regional expression of brain derived neurotrophic factor (BDNF) is correlated with dynamic patterns of promoter methylation in the developing mouse forebrain. Brain Res Mol Brain Res 140: 1–9.
Dutton RC, Maurer AJ, Sonner JM, Fanselow MS, Laster MJ, Eger 2nd EI (2001). The concentration of isoflurane required to suppress learning depends on the type of learning. Anesthesiology 94: 514–519.
Dykman RA, Ackerman PT, Newton JE (1997). Posttraumatic stress disorder: a sensitization reaction. Integr Physiol Behav Sci 32: 9–18.
Fanselow MS, Bolles RC (1979). Naloxone and shock-elicited freezing in the rat. J Comp Physiol Psychol 93: 736–744.
Fanselow MS, Lester LS (1988). A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD (eds). Evolution and Learning. Hillsdale: New Jersey. pp 185–211.
Flavell SW, Greenberg ME (2008). Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci 31: 563–590.
Galvez R, Mesches MH, McGaugh JL (1996). Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem 66: 253–257.
Ge H, Walhout AJ, Vidal M (2003). Integrating ‘omic’ information: a bridge between genomics and systems biology. Trends Genet 19: 551–560.
Huang DW, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J et al (2007). DAVID gene functional classification tool: a novel biological module-centric algorithm to functionally analyze large gene list. Genome Biol 8: R183.
Keeley MB, Wood MA, Isiegas C, Stein J, Hellman K, Hannenhalli S et al (2006). Differential transcriptional response to nonassociative and associative components of classical fear conditioning in the amygdala and hippocampus. Learn Mem 13: 135–142.
Lamprecht R, Dracheva S, Assoun S, Ledoux JE (2009). Fear conditioning induces distinct patterns of gene expression in lateral amygdala. Genes Brain Behav 8: 735–743.
Loepke AW, Istaphanous GK, McAuliffe 3rd JJ, Miles L, Hughes EA, McCann JC et al (2009). The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg 108: 90–104.
Lubin FD, Roth TL, Sweatt JD (2008). Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci 28: 10576–10586.
Maier SF, Grahn RE, Kalman BA, Sutton LC, Wiertelak EP, Watkins LR (1993). The role of the amygdala and dorsal raphe nucleus in mediating the behavioral consequences of inescapable shock. Behav Neurosci 107: 377–388.
McCool BA, Farroni JS (2001). Subunit composition of strychnine-sensitive glycine receptors expressed by adult rat basolateral amygdala neurons. Eur J Neurosci 14: 1082–1090.
Mei B, Li C, Dong S, Jiang CH, Wang H, Hu Y (2005). Distinct gene expression profiles in hippocampus and amygdala after fear conditioning. Brain Res Bull 67: 1–12.
Muotri AR, Chu VT, Marchetto MC, Deng W, Moran JV, Gage FH (2005). Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature 435: 903–910.
Oldham MC, Konopka G, Iwamoto K, Langfelder P, Kato T, Horvath S et al (2008). Functional organization of the transcriptome in human brain. Nat Neurosci 11: 1271–1282.
Ponomarev I, Maiya R, Harnett MT, Schafer GL, Ryabinin AE, Blednov YA et al (2006). Transcriptional signatures of cellular plasticity in mice lacking the alpha1 subunit of GABAA receptors. J Neurosci 26: 5673–5683.
Rampil IJ, Moller DH, Bell AH (2006). Isoflurane modulates genomic expression in rat amygdala. Anesth Analg 102: 1431–1438.
Ranft A, Kurz J, Deuringer M, Haseneder R, Dodt HU, Zieglgänsberger W et al (2004). Isoflurane modulates glutamatergic and GABAergic neurotransmission in the amygdala. Eur J Neurosci 20: 1276–1280.
Rau V, DeCola JP, Fanselow MS (2005). Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev 29: 1207–1223.
Rau V, Fanselow MS (2009). Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress 12: 125–133.
Rau V, Oh I, Laster M, Eger 2nd EI, Fanselow M (2009). Isoflurane suppresses stress-enhanced fear learning in a rodent model of post-traumatic stress disorder. Anesthesiology 110: 487–495.
Reijmers LG, Perkins BL, Matsuo N, Mayford M (2007). Localization of a stable neural correlate of associative memory. Science 317: 1230–1233.
Roozendaal B, McEwen BS, Chattarji S (2009). Stress, memory and the amygdala. Nat Rev Neurosci 10: 423–433.
Rosen JB, Schulkin J (1998). From normal fear to pathological anxiety. Psychol Rev 105: 325–350.
Roth TL, Sweatt JD (2009). Regulation of chromatin structure in memory formation. Curr Opin Neurobiol 19: 336–342.
Shin LM, Liberzon I (2009). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35: 169–191.
Slotkin RK, Martienssen R (2007). Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet 8: 272–285.
Smith MA, Weiss SR, Berry RL, Zhang LX, Clark M, Massenburg G et al (1997). Amygdala-kindled seizures increase the expression of corticotropin-releasing factor (CRF) and CRF-binding protein in GABAergic interneurons of the dentate hilus. Brain Res 745: 248–256.
Sosulina L, Meis S, Seifert G, Steinhäuser C, Pape HC (2006). Classification of projection neurons and interneurons in the rat lateral amygdala based upon cluster analysis. Mol Cell Neurosci 33: 57–67.
Storey JD (2002). A direct approach to false discovery rates. J Roy Stat Soc B 64: 479–498.
Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C et al (2006). Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci 9: 99–107.
Volterra A, Meldolesi J (2005). Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6: 626–640.
Waddell J, Bangasser DA, Shors TJ (2008). The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. J Neurosci 28: 5290–5294.
Acknowledgements
We thank Elizabeth Kahanek and Irene Oh for valuable technical assistance and Dr Jim Sonner for helpful discussion. This research was supported by the National Institute of Health Grants GM47818 (to RAH and EIE) and NIMH62122 (to MSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Ponomarev, I., Rau, V., Eger, E. et al. Amygdala Transcriptome and Cellular Mechanisms Underlying Stress-Enhanced Fear Learning in a Rat Model of Posttraumatic Stress Disorder. Neuropsychopharmacol 35, 1402–1411 (2010). https://doi.org/10.1038/npp.2010.10
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2010.10
Keywords
This article is cited by
-
Refinement of the stress-enhanced fear learning model of post-traumatic stress disorder: a behavioral and molecular analysis
Lab Animal (2022)
-
Past experience shapes the neural circuits recruited for future learning
Nature Neuroscience (2021)
-
Region- and time-dependent gene regulation in the amygdala and anterior cingulate cortex of a PTSD-like mouse model
Molecular Brain (2019)
-
Association of Serotonin Transporter Gene AluJb Methylation with Major Depression, Amygdala Responsiveness, 5-HTTLPR/rs25531 Polymorphism, and Stress
Neuropsychopharmacology (2018)
-
Induction and Expression of Fear Sensitization Caused by Acute Traumatic Stress
Neuropsychopharmacology (2016)