Abstract
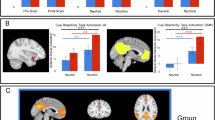
Attentional bias for drug-related stimuli, as measured by emotional Stroop (ES) tasks, is predictive of treatment outcomes for tobacco smoking and other abused drugs. Characterizing relationships between smoking-related attentional bias and brain reactivity to smoking images may help in identifying neural substrates critical to relapse vulnerability. To this end, we investigated putative relationships between interference effects in an offline smoking ES task and functional MRI (fMRI) measures of brain reactivity to smoking vs neutral images in women smokers. Positive correlations were found between attentional bias and reactivity to smoking images in brain areas involved in emotion, memory, interoception, and visual processing, including the amygdala, hippocampus, parahippocampal gyrus, insula, and occipital cortex. These findings suggest that smokers with elevated attentional biases to smoking-related stimuli may more readily shift attention away from other external stimuli and toward smoking stimuli-induced internal states and emotional memories. Such attentional shifts may contribute to increased interference by smoking cues, possibly increasing relapse vulnerability. Treatments capable of inhibiting shifts to drug cue-induced memories and internal states may lead to personalized tobacco dependence treatment for smokers with high attentional bias to smoking-related stimuli.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Amaral DG, Behniea H, Kelly JL (2003). Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience 118: 1099–1120.
Andreano JM, Cahill L (2009). Sex influences on the neurobiology of learning and memory. Learn Mem 16: 248–266.
Britton JC, Gold AL, Deckersbach T, Rauch SL (2009). Functional MRI study of specific animal phobia using an event-related emotional counting stroop paradigm. Depress Anxiety 26: 796–805.
Brody AL, Mandelkern MA, London ED, Childress AR, Lee G, Bota R et al (2002). Brain metabolic changes during cigarette craving. Arch Gen Psychiatry 59: 1162–1172.
Bush G, Shin LM (2006). The multi-source interference task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat Protoc 1: 308–313.
Cabeza R, Nyberg L (2000). Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47.
Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C et al (2001). Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem 75: 1–9.
Carpenter KM, Schreiber E, Church S, McDowell D (2006). Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav 31: 174–181.
Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL (2000). Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3: 292–297.
Cox LS, Tiffany ST, Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 3: 7–16.
Cox WM, Hogan LM, Kristian MR, Race JH (2002). Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug Alcohol Depend 68: 237–243.
Craig AD (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666.
Craig ADB (2009). How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59.
Due DL, Huettel SA, Hall WG, Rubin DC (2002). Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry 159: 954–960.
Eichenbaum H, Yonelinas AP, Ranganath C (2007). The medial temporal lobe and recognition memory. Annu Rev Neurosci 30: 123–152.
Etter J-F, Stapleton JA (2006). Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control 15: 280–285.
Fagerstrom KO (1978). Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 3: 235–241.
Ferguson SG, Shiffman S (2009). The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat 36: 235–243.
Field M, Duka T (2004). Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol Biochem Behav 78: 647–652.
Fiore MC, Smith SS, Jorenby DE, Baker TB (1994). The effectiveness of the nicotine patch for smoking cessation. A meta-analysis. JAMA 271: 1940–1947.
Fortin NJ, Wright SP, Eichenbaum H (2004). Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature 431: 188–191.
Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y et al (2007). Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology 32: 2301–2309.
Freed PJ, Yanagihara TK, Hirsch J, Mann JJ (2009). Neural mechanisms of grief regulation. Biol Psychiatry 66: 33–40.
Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B (1992). Predictors of smoking relapse among self-quitters: a report from the Normative Aging Study. Addict Behav 17: 367–377.
Gilbert DG, Rabinovich NE (1999). International Smoking Image Series (with Neutral Counterparts). version 1.2. Integrative Neuroscience Laboratory, Department of Psychology, Southern Illinois University.
Gross TM, Jarvik ME, Rosenblatt MR (1993). Nicotine abstinence produces content-specific Stroop interference. Psychopharmacology 110: 333–336.
Hamilton M (1967). Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6: 278–296.
Holmes AJ, Pizzagalli DA (2008). Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry 65: 179–188.
Janes AC, Frederick BD, Richardt S, Burbridge C, Merlo-Pich E, Renshaw PF et al (2009). Brain fMRI reactivity to smoking-related images before and during extended smoking abstinence. Exp Clin Psychopharmacol 17: 365–373.
Janes AC, Pizzagalli DA, Richardt S, Frederick BD, Chuzi S, Pachas G et al (2010). Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry 67: 722–729.
Kentridge RW, Shaw C, Aggleton JP (1991). Amygdaloid lesions and stimulus-reward associations in the rat. Behav Brain Res 42: 57–66.
Kirwan CB, Stark CEL (2004). Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus 14: 919–930.
MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838.
Manns JR, Eichenbaum H (2006). Evolution of declarative memory. Hippocampus 16: 795–808.
Marissen MAE, Franken IHA, Waters AJ, Blanken P, van den Brink W, Hendriks VM (2006). Attentional bias predicts heroin relapse following treatment. Addiction 101: 1306–1312.
McClernon F, Kozink RV, Rose JE (2008). Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology 33: 2148–2157.
Mitchell RLC (2005). The BOLD response during Stroop task-like inhibition paradigms: effects of task difficulty and task-relevant modality. Brain Cogn 59: 23–37.
Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007). Damage to the insula disrupts addiction to cigarette smoking. Science 315: 531–534.
Perkins K (2009). Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction 104: 1610–1616.
Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchinson S (2001). Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res 3: 141–150.
Phelps EA (2004). Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol 14: 198–202.
Phillips ML, Drevets WC, Rauch SL, Lane R (2003). Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry 54: 504–514.
Piefke M, Weiss PH, Markowitsch HJ, Fink GR (2005). Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Hum Brain Mapp 24: 313–324.
Ross RS, Slotnick SD (2008). The hippocampus is preferentially associated with memory for spatial context. J Cogn Neurosci 20: 432–446.
Santa Ana EJ, Rounsaville BJ, Frankforter TL, Nich C, Babuscio T, Poling J et al (2009). D-cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend 104: 220–227.
See RE, Fuch RA, Ledford CC, McLaughlin J (2003). Drug addiction, relapse, and the amygdala. Ann NY Acad Sci 985: 294–307.
Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M (1996). First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol 64: 366–379.
Slotnick SD, Moo LR, Segal JB, Hart J (2003). Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn Brain Res 17: 75–82.
Talairach J, Tournoux P (1988). Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers: New York.
Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH (2003). Attentional bias predicts outcome in smoking cessation. Health psychology: Official Journal of the Division of Health Psychology, American Psychological Association 22: 378–387.
Williams JM, Mathews A, MacLeod C (1996). The emotional Stroop task and psychopathology. Psychol Bull 120: 3–24.
World Health Organization (2008). WHO Report on the Global Tobacco Epidemic, 2008.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DAP: research support from ANT North America Inc. (Advanced Neuro Technology), consulting fees from ANT North America Inc. (Advanced Neuro Technology), and AstraZeneca for projects unrelated to the current study, and honoraria from AstraZeneca. AEE: research product support from Pfizer, and Speaker Honoraria from Reed Medical Education. MF: research support from Abbott Laboratories, Alkermes, Aspect Medical Systems, AstraZeneca, BioResearch, BrainCells, Inc., Bristol-Myers Squibb Company, Cephalon, Clinical Trial Solutions, LLC, Eli Lilly & Company, Forest Pharmaceuticals Inc., Ganeden, GlaxoSmithKline, J & J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, NARSAD, NCCAM, NIDA, NIMH, Novartis, Organon Inc., PamLab, LLC, Pfizer Inc., Pharmavite, Roche, Sanofi-Aventis, Shire, Solvay Pharmaceuticals, Inc., Synthelabo, and Wyeth-Ayerst Laboratories.
Advisory/consulting: Abbott Laboratories, Amarin, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer AG, Best Practice Project Management, Inc, BioMarin Pharmaceuticals, Inc., Biovail Pharmaceuticals, Inc., BrainCells, Inc, Bristol-Myers Squibb Company, Cephalon, Clinical Trials Solutions,LLC, CNS Response, Compellis, Cypress Pharmaceuticals, Dov Pharmaceuticals, Eisai, Inc., Eli Lilly & Company, EPIX Pharmaceuticals, Euthymics Bioscience, Inc., Fabre-Kramer, Pharmaceuticals, Inc., Forest Pharmaceuticals Inc., GlaxoSmithKline, Grunenthal GmBH, Janssen Pharmaceutica, Jazz Pharmaceuticals, J & J Pharmaceuticals, Knoll Pharmaceutical Company, Labopharm, Lorex Pharmaceuticals, Lundbeck, MedAvante Inc., Merck, Methylation Sciences, Neuronetics, Novartis, Nutrition 21, Organon Inc., PamLab, LLC, Pfizer Inc., PharmaStar, Pharmavite, Precision Human Biolaboratory, PsychoGenics, Psylin Neurosciences, Inc., Ridge Diagnostics, Inc., Roche, Sanofi-Aventis, Sepracor, Schering-Plough, Solvay Pharmaceuticals, Inc., Somaxon, Somerset Pharmaceuticals, Synthelabo, Takeda, Tetragenex, TransForm Pharmaceuticals, Inc., Transcept Pharmaceuticals, Vanda Pharmaceuticals Inc., and Wyeth-Ayerst Laboratories. Speaking/publishing: Adamed, Co., Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir, Boehringer-Ingelheim, Bristol-Myers Squibb Company, Cephalon, Eli Lilly & Company, Forest Pharmaceuticals Inc., GlaxoSmithKline, Imedex, Novartis, Organon Inc., Pfizer Inc, PharmaStar, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed-Elsevier, UBC, and Wyeth-Ayerst Laboratories.
Equity holdings: Compellis
Royalty/patent, other income: patent applications for SPCD and for a combination of azapirones and bupropion in MDD, copyright royalties for the MGH CPFQ, SFI, ATRQ, DESS, and SAFER
MJK: research support from GSK, Organon, Varian Inc., Advisory/consulting: Amgen, Novartis.
ACJ, BBF, SR, AJH, and JS declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Janes, A., Pizzagalli, D., Richardt, S. et al. Neural Substrates of Attentional Bias for Smoking-Related Cues: An fMRI Study. Neuropsychopharmacol 35, 2339–2345 (2010). https://doi.org/10.1038/npp.2010.103
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2010.103
Keywords
This article is cited by
-
Sex differences in mouse infralimbic cortex projections to the nucleus accumbens shell
Biology of Sex Differences (2023)
-
Activation of G protein-coupled estradiol receptor 1 in the dorsolateral striatum enhances motivation for cocaine and drug-induced reinstatement in female but not male rats
Biology of Sex Differences (2021)
-
Orbitofrontal cortex is selectively activated in a primate model of attentional bias to cocaine cues
Neuropsychopharmacology (2020)
-
ADHD, Smoking Withdrawal, and Inhibitory Control: Results of a Neuroimaging Study with Methylphenidate Challenge
Neuropsychopharmacology (2018)
-
Nicotine deprivation elevates neural representation of smoking-related cues in object-sensitive visual cortex: a proof of concept study
Psychopharmacology (2017)