Abstract
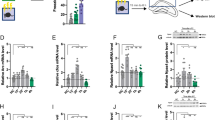
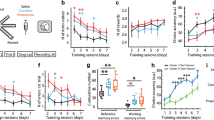
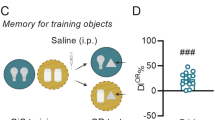
Neuropeptide S (NPS) has been shown to promote arousal and anxiolytic-like effects, as well as facilitation of fear extinction. In rodents, NPS receptors (NPSR) are prominently expressed in brain structures involved in learning and memory. Here, we investigate whether exogenous or endogenous NPS signaling can modulate acquisition, consolidation, or recall of emotional, spatial, and contextual memory traces, using two common behavioral paradigms, inhibitory avoidance (IA) and novel object recognition. In the IA paradigm, immediate and delayed post-training central NPS administration dose dependently enhanced memory retention in mice, indicating that NPS may act during the consolidation phase to enhance long-term memory. In contrast, pre-training or pre-test NPS injections were ineffective, suggesting that NPS had no effect on IA memory acquisition or recall. Peripheral administration of a synthetic NPSR antagonist attenuated NPS-induced IA memory enhancement, showing pharmacological specificity. NPS also enhanced hippocampal-dependent non-aversive memory in the novel object recognition task. In contrast, NPSR knockout mice displayed deficits in IA memory, novel object recognition, and novel place or context recognition, suggesting that activity of the endogenous NPS system is required for memory formation. Blockade of adrenergic signaling by propranolol attenuated NPS-induced memory enhancement in the IA task, indicating involvement of central noradrenergic systems. These results provide evidence for a facilitatory role of NPS in long-term memory, independent of memory content, possibly by acting as a salience signal or as an arousal-promoting factor.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Allen IC, Pace AJ, Jania LA, Ledford JG, Latour AM, Snouwaert JN et al (2006). Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am J Physiol Lung Cell Mol Physiol 291: L1005–L1017.
Aston-Jones G (2005). Brain structures and receptors involved in alertness. Sleep Med 6 (Suppl 1): S3–S7.
Broadbent NJ, Squire LR, Clark RE (2004). Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA 101: 14515–14520.
Cahill L, Brioni J, Izquierdo I (1986). Retrograde memory enhancement by diazepam: its relation to anterograde amnesia and some clinical implications. Psychopharmacology 90: 554–556.
Castellano C, Cabib S, Puglisi-Allegra S, Gasbarri A, Sulli A, Pacitti C et al (1999). Strain-dependent involvement of D1 and D2 dopamine receptors in muscarinic cholinergic influences on memory storage. Behav Brain Res 98: 17–26.
Chung S, Pohl S, Zeng J, Civelli O, Reinscheid RK (2006). Endogenous orphanin FQ/nociceptin is involved in the development of morphine tolerance. J Pharmacol Exp Ther 318: 262–267.
Clark RE, Zola SM, Squire LR (2000). Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20: 8853–8860.
Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N et al (1997). Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology 132: 107–124.
Deadwyler SA, Hampson RE (2004). Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron 42: 465–476.
Dornelles A, de Lima MN, Grazziotin M, Presti-Torres J, Garcia VA, Scalco FS et al (2007). Adrenergic enhancement of consolidation of object recognition memory. Neurobiol Learn Mem 88: 137–142.
Duangdao DM, Clark SD, Okamura N, Reinscheid RK (2009). Behavioral phenotyping of neuropeptide S receptor knockout mice. Behav Brain Res 205: 1–9.
Ferry B, Roozendaal B, McGaugh JL (1999). Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala. Biol Psychiatry 46: 1140–1152.
Gold PE, van Buskirk R (1975). Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behav Biol 13: 145–153.
Grillon C, Cordova J, Morgan CA, Charney DS, Davis M (2004). Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology 175: 342–352.
Introini-Collison I, Saghafi D, Novack GD, McGaugh JL (1992). Memory-enhancing effects of post-training dipivefrin and epinephrine: involvement of peripheral and central adrenergic receptors. Brain Res 572: 81–86.
Introini-Collison IB, Castellano C, McGaugh JL (1994). Interaction of GABAergic and beta-noradrenergic drugs in the regulation of memory storage. Behav Neural Biol 61: 150–155.
Izquierdo I, Dias RD (1983). The influence of adrenergic receptor antagonists on the amnestic and antiamnestic actions of adrenaline and tyramine. Psychopharmacology 80: 181–183.
Izquierdo I, Quillfeldt JA, Zanatta MS, Quevedo J, Schaeffer E, Schmitz PK et al (1997). Sequential role of hippocampus and amygdala, entorhinal cortex and parietal cortex in formation and retrieval of memory for inhibitory avoidance in rats. Eur J Neurosci 9: 786–793.
Jensen RA, Martinez JL, Vasquez BJ, McGaugh JL (1979). Benzodiazepines alter acquisition and retention of an inhibitory avoidance response in mice. Psychopharmacology 64: 125–126.
Jüngling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD et al (2008). Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron 59: 298–310.
Laursen SE, Belknap JK (1986). Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods 16: 355–357.
Leonard SK, Dwyer JM, Sukoff Rizzo SJ, Platt B, Logue SF, Neal SJ et al (2008). Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology 197: 601–611.
Liang KC, Juler RG, McGaugh JL (1986). Modulating effects of post-training epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res 368: 125–133.
Maren S, Quirk GJ (2004). Neuronal signalling of fear memory. Nat Rev Neurosci 5: 844–852.
McGaugh JL (1966). Time-dependent processes in memory storage. Science 153: 1351–1358.
McGaugh JL (2000). Memory—a century of consolidation. Science 287: 248–251.
McGaugh JL (2002). Memory consolidation and the amygdala: a systems perspective. Trends Neurosci 25: 456–461.
McGaugh JL, Roozendaal B (2009). Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology 202: 3–14.
Meis S, Bergado-Acosta JR, Yanagawa Y, Obata K, Stork O, Munsch T (2008). Identification of a neuropeptide S responsive circuitry shaping amygdala activity via the endopiriform nucleus. PLoS One 3: e2695.
Moses SN, Cole C, Driscoll I, Ryan JD (2005). Differential contributions of hippocampus, amygdala and perirhinal cortex to recognition of novel objects, contextual stimuli and stimulus relationships. Brain Res Bull 67: 62–76.
National Research Council (2003). Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. National Academies Press: Washington, DC.
Nielson KA, Czech DA, Laubmeier KK (1999). Chronic administration of propranolol impairs inhibitory avoidance retention in mice. Neurobiol Learn Mem 71: 248–257.
Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK (2008). Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J Pharmacol Exp Ther 325: 893–901.
Okamura N, Hashimoto K, Iyo M, Shimizu E, Dempfle A, Friedel S et al (2007). Gender-specific association of a functional coding polymorphism in the neuropeptide S receptor gene with panic disorder but not with schizophrenia or attention-deficit/hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry 31: 1444–1448.
Okamura N, Reinscheid RK (2007). Neuropeptide S: a novel modulator of stress and arousal. Stress 10: 221–226.
O’Mara S (2006). Controlling hippocampal output: the central role of subiculum in hippocampal information processing. Behav Brain Res 174: 304–312.
Pape HC, Paré D (2010). Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463.
Patel JB, Ciofalo VB, Iorio LC (1979). Benzodiazepine blockade of passive-avoidance task in mice: a state-dependent phenomenon. Psychopharmacology 61: 25–28.
Phelps EA, LeDoux JE (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48: 175–187.
Quirarte GL, Roozendaal B, McGaugh JL (1997). Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA 94: 14048–14053.
Reinscheid RK (2007). Phylogenetic appearance of neuropeptide S precursor proteins in tetrapods. Peptides 28: 830–837.
Reinscheid RK, Xu YL, Okamura N, Zeng J, Chung S, Pai R et al (2005). Pharmacological characterization of human and murine neuropeptide S receptor variants. J Pharmacol Exp Ther 315: 1338–1345.
Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S et al (2008). Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br J Pharmacol 154: 471–479.
Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL (2008). Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem 90: 576–579.
Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M et al (1999). Genetic enhancement of learning and memory in mice. Nature 401: 63–69.
Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ (2004). Double Dissociation between the effects of peri–postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci 24: 5901–5908.
Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK (2007). Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol 500: 84–102.
Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH et al (2004). Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron 43: 487–497.
Acknowledgements
We gratefully acknowledge scientific support by Drs Benno Roozendaal and Marcelo Wood (University of California, Irvine, CA), Natalie Rodrigue (Creascience, Montreal, Canada) for help with statistical analysis, and Dr Beverly H Koller (University of North Carolina at Chapel Hill) for making NPSR knockout mice available. This work was supported in part by a National Institute of Mental Health Grant (MH-71313; RKR), a postdoctoral fellowship from the Mitsubishi Pharma Research Foundation (NO), a training grant fellowship from the National Institute on Drug Abuse (DMD), a post-doctoral fellowship from the Canadian Institutes of Health Research (SDC), and a grant from the Deutsche Forschungsgemeinschaft (SFB-TRR58, TP A03; HCP), the Max-Planck Society, and Humboldt Foundation (Research Award 2007; HCP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Okamura, N., Garau, C., Duangdao, D. et al. Neuropeptide S Enhances Memory During the Consolidation Phase and Interacts with Noradrenergic Systems in the Brain. Neuropsychopharmacol 36, 744–752 (2011). https://doi.org/10.1038/npp.2010.207
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2010.207
Keywords
This article is cited by
-
Peroxisome proliferator-activated receptor-α activation facilitates contextual fear extinction and modulates intrinsic excitability of dentate gyrus neurons
Translational Psychiatry (2023)
-
Brain neuropeptide S: via GPCR activation to a powerful neuromodulator of socio-emotional behaviors
Cell and Tissue Research (2019)
-
Central noradrenergic activity affects analgesic effect of Neuropeptide S
Journal of Anesthesia (2018)
-
Protective effect of valproic acid in streptozotocin-induced sporadic Alzheimer’s disease mouse model: possible involvement of the cholinergic system
Naunyn-Schmiedeberg's Archives of Pharmacology (2017)
-
Exploring the role of neuropeptide S in the regulation of arousal: a functional anatomical study
Brain Structure and Function (2016)