Abstract
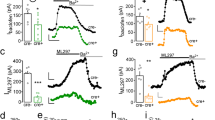
Atomoxetine and reboxetine are commonly used as selective norepinephrine reuptake inhibitors (NRIs) for the treatment of attention-deficit/hyperactivity disorder and depression, respectively. Furthermore, recent studies have suggested that NRIs may be useful for the treatment of several other psychiatric disorders. However, the molecular mechanisms underlying the various effects of NRIs have not yet been sufficiently clarified. G-protein-activated inwardly rectifying K+ (GIRK or Kir3) channels have an important function in regulating neuronal excitability and heart rate, and GIRK channel modulation has been suggested to be a potential treatment for several neuropsychiatric disorders and cardiac arrhythmias. In this study, we investigated the effects of atomoxetine and reboxetine on GIRK channels using the Xenopus oocyte expression assay. In oocytes injected with mRNA for GIRK1/GIRK2, GIRK2, or GIRK1/GIRK4 subunits, extracellular application of atomoxetine or reboxetine reversibly reduced GIRK currents. The inhibitory effects were concentration-dependent, but voltage-independent, and time-independent during each voltage pulse. However, Kir1.1 and Kir2.1 channels were insensitive to atomoxetine and reboxetine. Atomoxetine and reboxetine also inhibited GIRK currents induced by activation of cloned A1 adenosine receptors or by intracellularly applied GTPγS, a nonhydrolyzable GTP analogue. Furthermore, the GIRK currents induced by ethanol were concentration-dependently inhibited by extracellularly applied atomoxetine but not by intracellularly applied atomoxetine. The present results suggest that atomoxetine and reboxetine inhibit brain- and cardiac-type GIRK channels, revealing a novel characteristic of clinically used NRIs. GIRK channel inhibition may contribute to some of the therapeutic effects of NRIs and adverse side effects related to nervous system and heart function.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahern TH, Javors MA, Eagles DA, Martillotti J, Mitchell HA, Liles LC et al (2006). The effects of chronic norepinephrine transporter inactivation on seizure susceptibility in mice. Neuropsychopharmacology 31: 730–738.
Barker MJ, Benitez JG, Ternullo S, Juhl GA (2004). Acute oxcarbazepine and atomoxetine overdose with quetiapine. Vet Hum Toxicol 46: 130–132.
Belle DJ, Ernest CS, Sauer JM, Smith BP, Thomasson HR, Witcher JW (2002). Effect of potent CYP2D6 inhibition by paroxetine on atomoxetine pharmacokinetics. J Clin Pharmacol 42: 1219–1227.
Bettahi I, Marker CL, Roman MI, Wickman K (2002). Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh. J Biol Chem 277: 48282–48288.
Blednov YA, Stoffel M, Chang SR, Harris RA (2001). Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J Pharmacol Exp Ther 298: 521–530.
Boot J, Cases M, Clark BP, Findlay J, Gallagher PT, Hayhurst L et al (2005). Discovery and structure-activity relationships of novel selective norepinephrine and dual serotonin/norepinephrine reuptake inhibitors. Bioorg Med Chem Lett 15: 699–703.
Cruz HG, Berton F, Sollini M, Blanchet C, Pravetoni M, Wickman K et al (2008). Absence and rescue of morphine withdrawal in KIR/Kir3 knock-out mice. J Neurosci 28: 4069–4077.
Cusack B, Nelson A, Richelson E (1994). Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology 114: 559–565.
Dascal N (1997). Signalling via the G protein-activated K+ channels. Cell Signal 9: 551–573.
Fraser SP, Djamgoz MBA (1992). Xenopus oocytes: endogenous electrophysiological characteristics. In: Osborne NN (ed). Current Aspects of the Neurosciences, Vol 4. Macmillan: London. pp 267–315.
Garland M, Kirkpatrick P (2004). Atomoxetine hydrochloride. Nat Rev Drug Discovery 3: 385–386.
Garside D, Ropero-Miller JD, Riemer EC (2006). Postmortem tissue distribution of atomoxetine following fatal and nonfatal doses: three case reports. J Forensic Sci 51: 179–182.
Geller D, Donnelly C, Lopez F, Rubin R, Newcorn J, Sutton V et al (2007). Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 46: 1119–1127.
Hajós M, Fleishaker JC, Filipiak-Reisner JK, Brown MT, Wong EHF (2004). The selective norepinephrine reuptake inhibitor antidepressant reboxetine: pharmacological and clinical profile. CNS Drug Rev 10: 23–44.
Hashimoto N, Yamashita T, Tsuruzoe N (2006). Tertiapine, a selective IKACh blocker, terminates atrial fibrillation with selective atrial effective refractory period prolongation. Pharmacol Res 54: 136–141.
Hill KG, Alva H, Blednov YA, Cunningham CL (2003). Reduced ethanol-induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology 169: 108–114.
Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV et al (1993). Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362: 31–38.
Ikeda K, Yoshii M, Sora I, Kobayashi T (2003). Opioid receptor coupling to GIRK channels: in vitro studies using a Xenopus oocyte expression system and in vivo studies on weaver mutant mice. Methods Mol Med 84: 53–64.
Inanobe A, Yoshimoto Y, Horio Y, Morishige K-I, Hibino H, Matsumoto S et al (1999). Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci 19: 1006–1017.
Kadhe NG, Chillar AJ, Deshmukh YA (2003). Reboxetine: a novel antidepressant. J Postgrad Med 49: 373–375.
Kanegawa N, Kiyono Y, Kimura H, Sugita T, Kajiyama S, Kawashima H et al (2006). Synthesis and evaluation of radioiodinated (S,S)-2-(α-(2-iodophenoxy)benzyl)morpholine for imaging brain norepinephrine transporter. Eur J Nucl Med Mol Imaging 33: 639–647.
Karschin C, Dißmann E, Stuhmer W, Karschin A (1996). IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci 16: 3559–3570.
Keller NR, Diedrich A, Appalsamy M, Tuntrkool S, Lonce S, Finney C et al (2004). Norepinephrine transporter-deficient mice exhibit excessive tachycardia and elevated blood pressure with wakefulness and activity. Circulation 110: 1191–1196.
Kiyono Y, Kanegawa N, Kawashima H, Kitamura Y, Iida Y, Saji H (2004). Evaluation of radioiodinated (R)-N-methyl-3-(2-iodophenoxy)-3-phenylpropanamine as a ligand for brain norepinephrine transporter imaging. Nucl Med Biol 31: 147–153.
Kiyono Y, Sugita T, Ueda M, Kawashima H, Kanegawa N, Kuge Y et al (2008). Evaluation of radioiodinated (2S,αS)-2-(α-(2-iodophenoxy)benzyl)morpholine as a radioligand for imaging of norepinephrine transporter in the heart. Nucl Med Biol 35: 213–218.
Kobayashi T, Ikeda K (2006). G protein-activated inwardly rectifying potassium channels as potential therapeutic targets. Curr Pharm Des 12: 4513–4523.
Kobayashi T, Ikeda K, Ichikawa T, Abe S, Togashi S, Kumanishi T (1995). Molecular cloning of a mouse G-protein-activated K+ channel (mGIRK1) and distinct distributions of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem Biophys Res Commun 208: 1166–1173.
Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshioka T et al (1999). Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci 2: 1091–1097.
Kobayashi T, Ikeda K, Kumanishi T (2000). Inhibition by various antipsychotic drugs of the G-protein-activated inwardly rectifying K+ (GIRK) channels expressed in Xenopus oocytes. Br J Pharmacol 129: 1716–1722.
Kobayashi T, Ikeda K, Kumanishi T (2002). Functional characterization of an endogenous Xenopus oocyte adenosine receptor. Br J Pharmacol 135: 313–322.
Kobayashi T, Washiyama K, Ikeda K (2003). Inhibition of G protein-activated inwardly rectifying K+ channels by fluoxetine (Prozac). Br J Pharmacol 138: 1119–1128.
Kobayashi T, Washiyama K, Ikeda K (2006). Inhibition of G protein-activated inwardly rectifying K+ channels by the antidepressant paroxetine. J Pharmacol Sci 102: 278–287.
Kovoor A, Henry DJ, Chavkin C (1995). Agonist-induced desensitization of the mu opioid receptor-coupled potassium channel (GIRK1). J Biol Chem 270: 589–595.
Kovoor P, Wickman K, Maguire CT, Pu W, Gehrmann J, Berul CI et al (2001). Evaluation of the role of IKACh in atrial fibrillation using a mouse knockout model. J Am Coll Cardiol 37: 2136–2143.
Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE (1995). The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature 374: 135–141.
Kubo Y, Baldwin TJ, Jan YN, Jan LY (1993a). Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362: 127–133.
Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY (1993b). Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature 364: 802–806.
Kuzhikandathil EV, Oxford GS (2002). Classic D1 dopamine receptor antagonist R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) directly inhibits G protein-coupled inwardly rectifying potassium channels. Mol Pharmacol 62: 119–126.
Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M et al (1995). Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem 270: 28660–28667.
Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA (1999). G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci 2: 1084–1090.
Liao YJ, Jan YN, Jan LY (1996). Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci 16: 7137–7150.
LoVecchio F, Kashani J (2006). Isolated atomoxetine (STRATTERATM) ingestions commonly result in toxicity. J Emerg Med 31: 267–268.
Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA (1997). G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19: 687–695.
McElroy SL, Guerdjikova A, Kotwal R, Welge JA, Nelson EB, Lake KA et al (2007). Atomoxetine in the treatment of binge-eating disorder: a randomized placebo-controlled trial. J Clin Psychiatry 68: 390–398.
Montgomery SA (2005). Antidepressants and seizures: emphasis on newer agents and clinical implications. Int J Clin Pract 59: 1435–1440.
Morgan AD, Carroll ME, Loth AK, Stoffel M, Wickman K (2003). Decreased cocaine self-administration in Kir3 potassium channel subunit knockout mice. Neuropsychopharmacology 28: 932–938.
North RA (1989). Drug receptors and the inhibition of nerve cells. Br J Pharmacol 98: 13–28.
Öhman D, Norlander B, Peterson C, Bengtsson F (2001). Bioanalysis of racemic reboxetine and its desethylated metabolite in a therapeutic drug monitoring setting using solid phase extraction and HPLC. Ther Drug Monit 23: 27–34.
Poggesi I, Pellizzoni C, Fleishaker JC (2000). Pharmacokinetics of reboxetine in elderly patients with depressive disorders. Int J Clin Pharmacol Ther 38: 254–259.
Reimann F, Ashcroft FM (1999). Inwardly rectifying potassium channels. Curr Opin Cell Biol 11: 503–508.
Sawant S, Daviss SR (2004). Seizures and prolonged QTc with atomoxetine overdose. Am J Psychiatry 161: 757.
Scherer D, Hassel D, Bloehs R, Zitron E, von Löwenstern K, Seyler C et al (2009). Selective noradrenaline reuptake inhibitor atomoxetine directly blocks hERG currents. Br J Pharmacol 156: 226–236.
Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M (1997). Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci USA 94: 923–927.
Simpson D, Plosker GL (2004). Atomoxetine a review of its use in adults with attention deficit hyperactivity disorder. Drugs 64: 205–222.
Szerman N, Peris L, Mesías B, Colis P, Rosa J, Prieto A et al (2005). Reboxetine for the treatment of patients with cocaine dependence disorder. Hum Psychopharmacol Clin Exp 20: 189–192.
Takahashi T, Kobayashi T, Ozaki M, Takamatsu Y, Ogai Y, Ohta M et al (2006). G protein-activated inwardly rectifying K+ channel inhibition and rescue of weaver mouse motor functions by antidepressants. Neurosci Res 54: 104–111.
Tirado CF, Goldman M, Lynch K, Kampman KM, Obrien CP (2008). Atomoxetine for treatment of marijuana dependence: a report on the efficacy and high incidence of gastrointestinal adverse events in a pilot study. Drug Alcohol Depend 94: 254–257.
Versiani M, Cassano G, Perugi G, Benedetti A, Mastalli L, Nardi A et al (2002). Reboxetine, a selective norepinephrine reuptake inhibitor, is an effective and well-tolerated treatment for panic disorder. J Clin Psychiatry 63: 31–37.
Weber WM (1999). Ion currents of Xenopus laevis oocytes: state of the art. Biochim Biophys Acta 1421: 213–233.
Wernicke JF, Holdridge KC, Jin L, Edison T, Zhang S, Bangs ME et al (2007). Seizure risk in patients with attention-deficit-hyperactivity disorder treated with atomoxetine. Dev Med Child Neurol 49: 498–502.
Wilens TE, Adler LA, Weiss MD, Michelson D, Ramsey JL, Moore RJ et al (2008). Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend 96: 145–154.
Witcher JW, Long A, Smith B, Sauer JM, Heilgenstein J, Wilens T et al (2003). Atomoxetine pharmacokinetics in children and adolescents with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 13: 53–64.
Wong EHF, Sonders MS, Amara SG, Tinholt PM, Piercey MFP, Hoffmann WP et al (2000). Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol Psychiatry 47: 818–829.
Acknowledgements
We are grateful to Dr Kansaku Baba for his cooperation and Mr Kazuo Kobayashi (Niigata University) for his assistance. We also thank Dr Steven C Hebert and Dr Lily Y Jan for generously providing the Kir1.1 cDNA and Kir2.1 cDNA, respectively. This work was supported by research grants from the Ministry of Education, Science, Sports, and Culture of Japan and from the Ministry of Health, Labour, and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that over the past 3 years Kazutaka Ikeda has received research grants or expenses that are not related to this study from Fujifilm Corporation, the Mitsubishi Foundation, the Naito Foundation, and the Smoking Science Foundation, and a lecture fee from Dainippon Sumitomo Pharma and Kyowa Hakko Kirin. The authors declare that, except for income received from their primary employer and the aforementioned disclosures, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Kobayashi, T., Washiyama, K. & Ikeda, K. Inhibition of G-Protein-Activated Inwardly Rectifying K+ Channels by the Selective Norepinephrine Reuptake Inhibitors Atomoxetine and Reboxetine. Neuropsychopharmacol 35, 1560–1569 (2010). https://doi.org/10.1038/npp.2010.27
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2010.27
Keywords
This article is cited by
-
Atomoxetine produces oxidative stress and alters mitochondrial function in human neuron-like cells
Scientific Reports (2019)
-
Paroxetine suppresses recombinant human P2X7 responses
Purinergic Signalling (2015)
-
Layer- and Area-Specificity of the Adrenergic Modulation of Synaptic Transmission in the Rat Neocortex
Neurochemical Research (2014)
-
In vitro study methodologies to investigate genetic aspects and effects of drugs used in attention-deficit hyperactivity disorder
Journal of Neural Transmission (2013)