Abstract
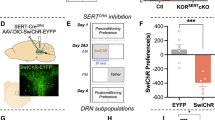
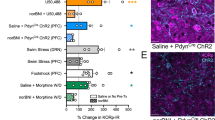
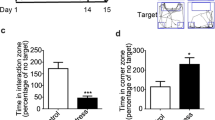
Stress exposure increases the risk of addictive drug use in human and animal models of drug addiction by mechanisms that are not completely understood. Mice subjected to repeated forced swim stress (FSS) before cocaine develop significantly greater conditioned place preference (CPP) for the drug-paired chamber than unstressed mice. Analysis of the dose dependency showed that FSS increased both the maximal CPP response and sensitivity to cocaine. To determine whether FSS potentiated CPP by enhancing associative learning mechanisms, mice were conditioned with cocaine in the absence of stress, then challenged after association was complete with the κ-opioid receptor (KOR) agonist U50,488 or repeated FSS, before preference testing. Mice challenged with U50,488 60 min before CPP preference testing expressed significantly greater cocaine–CPP than saline-challenged mice. Potentiation by U50,488 was dose and time dependent and blocked by the KOR antagonist norbinaltorphimine (norBNI). Similarly, mice subjected to repeated FSS before the final preference test expressed significantly greater cocaine–CPP than unstressed controls, and FSS-induced potentiation was blocked by norBNI. Novel object recognition (NOR) performance was not affected by U50,488 given 60 min before assay, but was impaired when given 15 min before NOR assay, suggesting that KOR activation did not potentiate CPP by facilitating memory retrieval or expression. The results from this study show that the potentiation of cocaine–CPP by KOR activation does not result from an enhancement of associative learning mechanisms and that stress may instead enhance the rewarding valence of cocaine-associated cues by a dynorphin-dependent mechanism.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahmed SH, Kenny PJ, Koob GF, Markou A (2002). Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci 5: 625–626.
Bardo MT, Rowlett JK, Harris MJ (1995). Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev 19: 39–51.
Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S et al (2007a). Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci 27: 11614–11623.
Bruchas MR, Land BB, Chavkin C (2010). The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314: 44–55.
Bruchas MR, Land BB, Lemos JC, Chavkin C (2009b). CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One 4: e8528.
Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S et al (2007b). Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem 282: 29803–29811.
Bruchas MR, Xu M, Chavkin C (2008). Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport 19: 1417–1422.
Caine SB, Negus SS, Mello NK (2000). Effects of dopamine D(1-like) and D(2-like) agonists on cocaine self-administration in rhesus monkeys: rapid assessment of cocaine dose-effect functions. Psychopharmacology (Berl) 148: 41–51.
Carey AN, Borozny K, Aldrich JV, McLaughlin JP (2007). Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol 569: 84–89.
Carey AN, Lyons AM, Shay CF, Dunton O, McLaughlin JP (2009). Endogenous kappa opioid activation mediates stress-induced deficits in learning and memory. J Neurosci 29: 4293–4300.
Carlezon Jr WA, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS et al (2006). Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther 316: 440–447.
Castellano C, Libri V, Ammassari-Teule M (1988). The amygdala mediates the impairing effect of the selective kappa-opioid receptor agonist U-50,488 on memory in CD1 mice. Behav Brain Res 30: 259–263.
Chavkin C (2000). Dynorphins are endogenous opioid peptides released from granule cells to act neurohumorly and inhibit excitatory neurotransmission in the hippocampus. Prog Brain Res 125: 363–367.
Chavkin C, Goldstein A (1981). Demonstration of a specific dynorphin receptor in guinea pig ileum myenteric plexus. Nature 291: 591–593.
Chavkin C, James IF, Goldstein A (1982). Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215: 413–415.
Covington III HE, Miczek KA (2005). Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 183: 331–340.
Daumas S, Betourne A, Halley H, Wolfer DP, Lipp HP, Lassalle JM et al (2007). Transient activation of the CA3 kappa opioid system in the dorsal hippocampus modulates complex memory processing in mice. Neurobiol Learn Mem 88: 94–103.
de Quervain DJ, Roozendaal B, McGaugh JL (1998). Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 394: 787–790.
Ennaceur A, Delacour J (1988). A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31: 47–59.
Goeders NE, Guerin GF (1994). Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 114: 63–70.
Gold PW, Chrousos GP (2002). Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 7: 254–275.
Haile CN, GrandPre T, Kosten TA (2001). Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology (Berl) 154: 213–220.
Haney M, Maccari S, Le Moal M, Simon H, Piazza PV (1995). Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res 698: 46–52.
Hiramatsu M, Hoshino T (2004). Involvement of kappa-opioid receptors and sigma receptors in memory function demonstrated using an antisense strategy. Brain Res 1030: 247–255.
Hiramatsu M, Watanabe E (2006). Dynorphin A (2–13) improves mecamylamine-induced learning impairment accompanied by reversal of reductions in acetylcholine release in rats. Neuropeptides 40: 47–56.
Knoll AT, Carlezon Jr WA (2010). Dynorphin, stress, and depression. Brain Res 1314: 56–73.
Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon Jr WA (2007). Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther 323: 838–845.
Koob G, Kreek MJ (2007). Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164: 1149–1159.
Koob GF, Le Moal M (2005). Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8: 1442–1444.
Koob GF, Le Moal M (2008). Addiction and the brain antireward system. Annu Rev Psychol 59: 29–53.
Kreibich AS, Briand L, Cleck JN, Ecke L, Rice KC, Blendy JA (2009). Stress-induced potentiation of cocaine reward: a role for CRF R1 and CREB. Neuropsychopharmacology 34: 2609–2617.
Kuzmin A, Madjid N, Terenius L, Ogren SO, Bakalkin G (2006). Big dynorphin, a prodynorphin-derived peptide produces NMDA receptor-mediated effects on memory, anxiolytic-like and locomotor behavior in mice. Neuropsychopharmacology 31: 1928–1937.
Kuzmin A, Sandin J, Terenius L, Ogren SO (2000). Dose- and time-dependent bimodal effects of kappa-opioid agonists on locomotor activity in mice. J Pharmacol Exp Ther 295: 1031–1042.
Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C (2008). The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci 28: 407–414.
Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D et al (2009). Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA 106: 19168–19173.
Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens Jr WC et al (2003). Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther 305: 323–330.
McGaugh JL, Cahill L, Roozendaal B (1996). Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci USA 93: 13508–13514.
McGaugh JL, Roozendaal B (2009). Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 202: 3–14.
McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C (2006a). Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology 31: 787–794.
McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C (2006b). Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology 31: 1241–1248.
McLaughlin JP, Marton-Popovici M, Chavkin C (2003). Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 23: 5674–5683.
Negus SS (2004). Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 176: 204–213.
Pfeiffer A, Brantl V, Herz A, Emrich HM (1986). Psychotomimesis mediated by kappa opiate receptors. Science 233: 774–776.
Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H (1990). Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol 1: 339–345.
Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon Jr WA (2001). Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21: 7397–7403.
Porsolt RD, Le Pichon M, Jalfre M (1977). Depression: a new animal model sensitive to antidepressant treatments. Nature 266: 730–732.
Redila VA, Chavkin C (2008). Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 200: 59–70.
Sanchez CJ, Sorg BA (2001). Conditioned fear stimuli reinstate cocaine-induced conditioned place preference. Brain Res 908: 86–92.
Sandin J, Nylander I, Georgieva J, Schott PA, Ogren SO, Terenius L (1998). Hippocampal dynorphin B injections impair spatial learning in rats: a kappa-opioid receptor-mediated effect. Neuroscience 85: 375–382.
Schwarzer C (2009). 30 years of dynorphins—new insights on their functions in neuropsychiatric diseases. Pharmacol Ther 123: 353–370.
Shaham Y, Erb S, Stewart J (2000). Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev 33: 13–33.
Shippenberg TS, Herz A (1986). Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr 75: 563–566.
Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ (2006). Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63: 324–331.
Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M (2008). Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci USA 105: 17145–17150.
Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon Jr WA (2008). The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry 64: 982–988.
Tzschentke TM (2007). Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12: 227–462.
Wagner JJ, Terman GW, Chavkin C (1993). Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature 363: 451–454.
Will MJ, Watkins LR, Maier SF (1998). Uncontrollable stress potentiates morphine's rewarding properties. Pharmacol Biochem Behav 60: 655–664.
Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS et al (2008). Effects of cocaine place conditioning, chronic escalating-dose ‘binge’ pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience 153: 1225–1234.
Acknowledgements
This work was supported by USPHS grants DA07278, DA25970, DA20570, and an award from the Hope for Depression Research Foundation. We thank Dan Messinger for genotyping the mice and maintaining the breeding colony. Dr Michael Garelick (University of Washington) and Dr Julie Blendy (University of Pennsylvania) provided helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A Schindler has no conflict of interest to disclose. S Li is currently employed by Astrazeneca, but had no conflict of interest during her participation in this study, which solely involved the generation of the data shown in Figure 2. C Chavkin has no conflict of interest or consulting relationships to disclose, but has received outside compensation for seminars on his NIH-funded research during the last 3 years at Astrazeneca Corp, Wilmington DE (2/25/08); National Institute on Drug Abuse, Bethesda, MD (2/26/08); Department of Pharmacology at the University of California, Irvine (6/9/08); Department of Pharmacology at the Uniformed Services University, Bethesda, MD (11/13/08); Neurobiology Program, University of Minnesota, Minneapolis, MN (4/24/09); Sepracor Corporation, Boston MA (6/12/09); Eli Lilly Corporation, Indianapolis, IN (10/27/09); Adolor Corporation, Exton, PA (11/5/09); Neurosciences Program at Vanderbilt University (2/17/10). He received a customary honorarium for each of these talks. In addition, C Chavkin served as a grant reviewer for NIH on 10/12/09, 1/8/10, and 3/10/10 and received a customary honorarium for his CSR-review committee service.
Rights and permissions
About this article
Cite this article
Schindler, A., Li, S. & Chavkin, C. Behavioral Stress May Increase the Rewarding Valence of Cocaine-Associated Cues Through a Dynorphin/κ-Opioid Receptor-Mediated Mechanism without Affecting Associative Learning or Memory Retrieval Mechanisms. Neuropsychopharmacol 35, 1932–1942 (2010). https://doi.org/10.1038/npp.2010.67
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2010.67
Keywords
This article is cited by
-
Dichotomous regulation of striatal plasticity by dynorphin
Molecular Psychiatry (2023)
-
Suicide Risk and Addiction: The Impact of Alcohol and Opioid Use Disorders
Current Addiction Reports (2021)
-
Sex differences in neural mechanisms mediating reward and addiction
Neuropsychopharmacology (2019)
-
Antireward, compulsivity, and addiction: seminal contributions of Dr. Athina Markou to motivational dysregulation in addiction
Psychopharmacology (2017)
-
Relative Timing Between Kappa Opioid Receptor Activation and Cocaine Determines the Impact on Reward and Dopamine Release
Neuropsychopharmacology (2016)