Abstract
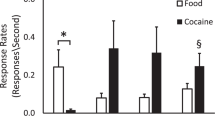
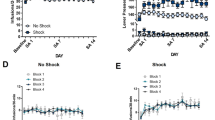
Metabotropic glutamate receptor 2/3 (mGluR2/3) agonists were shown previously to nonselectively decrease both cocaine- and food-maintained responding in rats. mGluR2 positive allosteric modulators (PAMs) may represent improved therapeutic compounds because of their modulatory properties and higher selectivity for mGluR2. We analyzed the effects of the selective, brain penetrant, and systemically active mGluR2 PAM potassium 3′-([(2-cyclopentyl-6-7-dimethyl-1-oxo-2,3-dihydro-1H-inden-5-yl)oxy]methyl)biphenyl l-4-carboxylate (BINA) and the mGluR2/3 agonist LY379268 on intravenous cocaine self-administration and cocaine-seeking behavior in rats that had short (1 h, ShA) or long (6 h, LgA) access to cocaine. The effects of BINA on food responding and food-seeking behavior were also analyzed. Finally, we examined the effects of BINA on brain reward function and cocaine-induced reward enhancement using the intracranial self-stimulation procedure. BINA decreased cocaine self-administration in both ShA and LgA rats, with no effect on food self-administration. Alternatively, LY379268 nonselectively decreased both cocaine and food self-administration. BINA decreased cue-induced reinstatement of cocaine seeking with no effect on food seeking. The cocaine-induced enhancement of brain reward function was blocked by BINA, although the highest doses of BINA decreased brain reward function when administered alone, suggesting additive, rather than interactive, effects of BINA and cocaine. In conclusion, BINA attenuated the reinforcing and counteracted the reward-enhancing effects of cocaine and decreased cue-induced cocaine-seeking behavior, without affecting behaviors motivated by food reinforcement. The higher selectivity of BINA compared with an mGluR2/3 agonist for drug- vs food-motivated behaviors suggests a therapeutic role for mGluR2 PAMs for the treatment of cocaine addiction and possibly other drugs of abuse.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adewale AS, Platt DM, Spealman RD (2006). Pharmacological stimulation of group II metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther 318: 922–931.
Ahmed SH, Cador M (2006). Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology 31: 563–571.
Ahmed SH, Kenny PJ, Koob GF, Markou A (2002). Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci 5: 625–626.
Ahmed SH, Koob GF (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science 282: 298–300.
Ahmed SH, Walker JR, Koob GF (2000). Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22: 413–421.
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Press: Washington, DC.
Anwyl R (1999). Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 29: 83–120.
Aujla H, Martin-Fardon R, Weiss F (2008). Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology 33: 1818–1826.
Backstrom P, Hyytia P (2005). Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol 528: 110–118.
Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S et al (2003). Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6: 743–749.
Baker DA, Shen H, Kalivas PW (2002). Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids 23: 161–162.
Baptista MA, Martin-Fardon R, Weiss F (2004). Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci 24: 4723–4727.
Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A (2005). Prolonged daily exposure to i.v. cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav 82: 411–416.
Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E (2007). A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol 72: 477–484.
Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E et al (2005). Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology 49 (Suppl 1): 167–178.
Bonnefous C, Vernier JM, Hutchinson JH, Gardner MF, Cramer M, James JK et al (2005). Biphenyl-indanones: allosteric potentiators of the metabotropic glutamate subtype 2 receptor. Bioorg Med Chem Lett 15: 4354–4358.
Bossert JM, Busch RF, Gray SM (2005). The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport 16: 1013–1016.
Bossert JM, Gray SM, Lu L, Shaham Y (2006a). Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology 31: 2197–2209.
Bossert JM, Liu SY, Lu L, Shaham Y (2004). A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci 24: 10726–10730.
Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE (2006b). The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res 173: 148–152.
Cartmell J, Monn JA, Schoepp DD (1999). The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther 291: 161–170.
Cartmell J, Monn JA, Schoepp DD (2000). Attenuation of specific PCP-evoked behaviors by the potent mGlu2/3 receptor agonist, LY379268 and comparison with the atypical antipsychotic, clozapine. Psychopharmacology (Berl) 148: 423–429.
Cartmell J, Schoepp DD (2000). Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75: 889–907.
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L et al (2006). Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 189: 27–36.
Conn PJ, Pin JP (1997). Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37: 205–237.
Dackis CA, O’Brien CP (2001). Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat 21: 111–117.
Epstein DH, Preston KL, Stewart J, Shaham Y (2006). Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189: 1–16.
Esposito RU, Motola AH, Kornetsky C (1978). Cocaine: acute effects on reinforcement thresholds for self-stimulation behavior to the medial forebrain bundle. Pharmacol Biochem Behav 8: 437–439.
Feltenstein MW, See RE (2008). The neurocircuitry of addiction: an overview. Br J Pharmacol 154: 261–274.
Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE (2005). Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry 58: 751–759.
Frank RA, Martz S, Pommering T (1988). The effect of chronic cocaine on self-stimulation train-duration thresholds. Pharmacol Biochem Behav 29: 755–758.
Galici R, Echemendia NG, Rodriguez AL, Conn PJ (2005). A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther 315: 1181–1187.
Galici R, Jones CK, Hemstapat K, Nong Y, Echemendia NG, Williams LC et al (2006). Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J Pharmacol Exp Ther 318: 173–185.
Gass JT, Olive MF (2008). Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol 75: 218–265.
Govek SP, Bonnefous C, Hutchinson JH, Kamenecka T, McQuiston J, Pracitto R et al (2005). Benzazoles as allosteric potentiators of metabotropic glutamate receptor 2 (mGluR2): efficacy in an animal model for schizophrenia. Bioorg Med Chem Lett 15: 4068–4072.
Harrison AA, Gasparini F, Markou A (2002). Nicotine potentiation of brain stimulation reward reversed by DHβE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 160: 56–66.
Hemstapat K, Da Costa H, Nong Y, Brady AE, Luo Q, Niswender CM et al (2007). A novel family of potent negative allosteric modulators of group II metabotropic glutamate receptors. J Pharmacol Exp Ther 322: 254–264.
Hu E, Chua PC, Tehrani L, Nagasawa JY, Pinkerton AB, Rowe BA et al (2004). Pyrimidine methyl anilines: selective potentiators for the metabotropic glutamate 2 receptor. Bioorg Med Chem Lett 14: 5071–5074.
Hyman SM, Garcia M, Sinha R (2006). Gender specific associations between types of childhood maltreatment and the onset, escalation and severity of substance use in cocaine dependent adults. Am J Drug Alcohol Abuse 32: 655–664.
Johnson BG, Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD (1999). [3H]-LY341495 as a novel antagonist radioligand for group II metabotropic glutamate (mGlu) receptors: characterization of binding to membranes of mGlu receptor subtype expressing cells. Neuropharmacology 38: 1519–1529.
Johnson MP, Baez M, Jagdmann Jr GE, Britton TC, Large TH, Callagaro DO et al (2003). Discovery of allosteric potentiators for the metabotropic glutamate 2 receptor: synthesis and subtype selectivity of N-(4-(2-methoxyphenoxy)phenyl)-N-(2,2,2-trifluoroethylsulfonyl)pyrid-3-ylmethylamine. J Med Chem 46: 3189–3192.
Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE et al (2005). Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s). Psychopharmacology (Berl) 179: 271–283.
Kalivas PW (2004). Glutamate systems in cocaine addiction. Curr Opin Pharmacol 4: 23–29.
Kalivas PW (2009). The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10: 561–572.
Kalivas PW, Duffy P (1998). Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem 70: 1497–1502.
Kalivas PW, Volkow ND (2005). The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162: 1403–1413.
Katz JL, Higgins ST (2003). The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 168: 21–30 [erratum: 168: 244].
Kenny PJ (2007). Brain reward systems and compulsive drug use. Trends Pharmacol Sci 28: 135–141.
Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF (2006). Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci 26: 5894–5900.
Kenny PJ, Markou A (2004). The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci 25: 265–272.
Kenny PJ, Markou A (2006). Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology 31: 1203–1211.
Kenny PJ, Polis I, Koob GF, Markou A (2003). Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur J Neurosci 17: 191–195.
Kim JH, Austin JD, Tanabe L, Creekmore E, Vezina P (2005). Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci 21: 295–300 [erratum: 25: 908].
Kippin TE, Fuchs RA, See RE (2006). Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 187: 60–67.
Knackstedt LA, Kalivas PW (2007). Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther 322: 1103–1109.
Knackstedt LA, Kalivas PW (2009). Glutamate and reinstatement. Curr Opin Pharmacol 9: 59–64.
Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S et al (2009). The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry 65: 841–845.
Knackstedt LA, Melendez RI, Kalivas PW (2010). Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry 67: 81–84.
Kokkinidis L, McCarter BD (1990). Postcocaine depression and sensitization of brain-stimulation reward: analysis of reinforcement and performance effects. Pharmacol Biochem Behav 36: 463–471.
Koob GF (2009). Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56 (Suppl 1): 18–31.
Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A et al (2004). Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev 27: 739–749.
Kornetsky C, Esposito RU, McLean S, Jacobson JO (1979). Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch Gen Psychiatry 36: 289–292.
Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD (2005). Attenuation of behavioral effects of cocaine by the metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther 312: 1232–1240.
Leshner AI (1997). Addiction is a brain disease, and it matters. Science 278: 45–47.
Liechti ME, Lhuillier L, Kaupmann K, Markou A (2007). Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci 27: 9077–9085.
Liechti ME, Markou A (2007). Metabotropic glutamate 2/3 receptor activation induced reward deficits but did not aggravate brain reward deficits associated with spontaneous nicotine withdrawal in rats. Biochem Pharmacol 74: 1299–1307.
Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y (2007). Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry 61: 591–598.
Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M et al (2007). Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci 27: 13968–13976.
Mansvelder HD, McGehee DS (2000). Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27: 349–357.
Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ (2004). Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 175: 26–36.
Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK (2000). Physiological antagonism between 5-hydroxytryptamine2A and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther 292: 76–87.
Markou A (2007). Metabotropic glutamate receptor antagonists: novel therapeutics for nicotine dependence and depression? Biol Psychiatry 61: 17–22.
Markou A, Koob GF (1992). Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav 51: 111–119.
Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF (1993). Animal models of drug craving. Psychopharmacology (Berl) 112: 163–182.
Melendez RI, Vuthiganon J, Kalivas PW (2005). Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther 314: 139–147.
Mello NK, Negus SS (1996). Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14: 375–424.
Mogenson GJ, Jones DL, Yim CY (1980). From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14: 69–97.
Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP et al (1999). Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem 42: 1027–1040.
Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK (2005). Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 25: 6389–6393.
Niswender CM, Johnson KA, Luo Q, Ayala JE, Kim C, Conn PJ et al (2008). A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Mol Pharmacol 73: 1213–1224.
O’Brien CP, Childress AR, Ehrman R, Robbins SJ (1998). Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12: 15–22.
O’Brien CP, Gardner EL (2005). Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther 108: 18–58.
O’Brien CP, McLellan AT (1996). Myths about the treatment of addiction. Lancet 347: 237–240.
Ohishi H, Neki A, Mizuno N (1998). Distribution of a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat and mouse: an immunohistochemical study with a monoclonal antibody. Neurosci Res 30: 65–82.
Paterson NE, Markou A (2003). Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport 14: 2229–2232.
Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A (2008). Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J Pharmacol Exp Ther 326: 306–314.
Pellegrino L, Pellegrino A, Cushman A (1986). A Stereotaxic Atlas of the Rat Brain, 2nd edn. Plenum Press: New York.
Peters J, Kalivas PW (2006). The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 186: 143–149.
Phillips AG, Fibiger HC (1990). Role of reward and enhancement of conditioned reward in persistence of responding for cocaine. Behav Pharmacol 1: 269–282.
Pinkerton AB, Cube RV, Hutchinson JH, Rowe BA, Schaffhauser H, Zhao X et al (2004). Allosteric potentiators of the metabotropic glutamate receptor 2 (mGlu2): part 1. Identification and synthesis of phenyl-tetrazolyl acetophenones. Bioorg Med Chem Lett 14: 5329–5332.
Rice ME, Cragg SJ (2004). Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci 7: 583–584.
Robbins TW, Watson BA, Gaskin M, Ennis C (1983). Contrasting interactions of pipradrol, d-amphetamine, cocaine, cocaine analogues, apomorphine and other drugs with conditioned reinforcement. Psychopharmacology (Berl) 80: 113–119.
Sanger DJ, Blackman DE (1976). Rate-dependent effects of drugs: a review of the literature. Pharmacol Biochem Behav 4: 73–83.
Schaffhauser H, Rowe BA, Morales S, Chavez-Noriega LE, Yin R, Jachec C et al (2003). Pharmacological characterization and identification of amino acids involved in the positive modulation of metabotropic glutamate receptor subtype 2. Mol Pharmacol 64: 798–810.
Schoepp DD (2001). Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299: 12–20.
Semenova S, Markou A (2003a). Cocaine-seeking behavior after extended cocaine-free periods in rats: role of conditioned stimuli. Psychopharmacology (Berl) 168: 192–200.
Semenova S, Markou A (2003b). Clozapine treatment attenuated somatic and affective signs of nicotine and amphetamine withdrawal in subsets of rats exhibiting hyposensitivity to the initial effects of clozapine. Biol Psychiatry 54: 1249–1264.
Shalev U, Grimm JW, Shaham Y (2002). Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54: 1–42.
Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG (2006). Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology (Berl) 184: 637–644.
Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW (2001). Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci 21: 9043–9052.
Tamaru Y, Nomura S, Mizuno N, Shigemoto R (2001). Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience 106: 481–503.
Taylor JR, Robbins TW (1986). 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology (Berl) 90: 390–397.
Ungless MA, Whistler JL, Malenka RC, Bonci A (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411: 583–587.
Vanderschuren LJ, Everitt BJ (2004). Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305: 1017–1019.
Weaver CD, Harden D, Dworetzky SI, Robertson B, Knox RJ (2004). A thallium-sensitive, fluorescence-based assay for detecting and characterizing potassium channel modulators in mammalian cells. J Biomol Screen 9: 671–677.
Weiss F (2005). Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol 5: 9–19.
Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN (2008). The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 196: 431–440.
Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW (2002a). Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther 300: 162–171.
Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW (2002b). Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther 303: 608–615.
Xie X, Steketee JD (2009). Effects of repeated exposure to cocaine on group II metabotropic glutamate receptor function in the rat medial prefrontal cortex: behavioral and neurochemical studies. Psychopharmacology (Berl) 203: 501–510.
Xie X, Steketee JD (2008). Repeated exposure to cocaine alters the modulation of mesocorticolimbic glutamate transmission by medial prefrontal cortex Group II metabotropic glutamate receptors. J Neurochem 107: 186–196.
Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F (2006). Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci 26: 9967–9974.
Acknowledgements
This work was supported by National Institutes of Health grant R01 DA023926 to NC. We thank Ms Kim Edwards and Mrs Jessica Benedict for technical assistance and Mr Michael Arends for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AM has received contract research support from Intracellular Therapeutics, Lundbeck Research USA, Bristol–Myers Squibb, F Hoffman-La Roche, Pfizer, and Astra-Zeneca and honorarium/consulting fees from Abbott GmbH and Company, AstraZeneca, and Pfizer during the past 3 years. AM and SS have a patent application on metabotropic glutamate receptors and drug dependence. PJC receives research support, including salary support, from Seaside Therapeutics and Johnson and Johnson and has received consulting fees or speaking honoraria from Eli Lilly and Company, Invitrogen, Roche Pharmaceutical, Cephalon, AstraZeneca, Bristol–Myers Squibb, Addex, Forest Research Institute, LEK Consulting, Merck and Company, Epix Pharmaceuticals, AMRI, Evotec, Millipore, Genentech, IMS Health, Sepracor, Seaside Therapeutics, Lundbeck Research, Otsuka Pharmaceuticals, Prestwich Pharmaceuticals, Primary Insight, The Frankel Group, Metastatix, GlaxoSmithKline, Adolor, Abbott Laboratories, Merck Serono, Johnson and Johnson, Solvay, and PureTech during the past 3 years. PJC is an inventor on multiple patents and pending patent applications on specific allosteric modulators of GPCRs, none of which is included in this study. NC has received research support from Brain Cells and honorarium/consulting fees from Vertex Pharmaceuticals during the past 3 years. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Jin, X., Semenova, S., Yang, L. et al. The mGluR2 Positive Allosteric Modulator BINA Decreases Cocaine Self-Administration and Cue-Induced Cocaine-Seeking and Counteracts Cocaine-Induced Enhancement of Brain Reward Function in Rats. Neuropsychopharmacol 35, 2021–2036 (2010). https://doi.org/10.1038/npp.2010.82
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2010.82
Keywords
This article is cited by
-
Positive allosteric mGluR2 modulation with BINA alleviates dyskinesia and psychosis-like behaviours in the MPTP-lesioned marmoset
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Operant self-stimulation of thalamic terminals in the dorsomedial striatum is constrained by metabotropic glutamate receptor 2
Neuropsychopharmacology (2020)
-
Improving translation of animal models of addiction and relapse by reverse translation
Nature Reviews Neuroscience (2020)
-
Effects of the mGluR2/3 receptor agonist LY379268 on the reinforcing strength of cocaine in rhesus monkeys
Psychopharmacology (2020)
-
From bench to bedside: mGluR2 positive allosteric modulators as medications to treat substance use disorders
Psychopharmacology (2017)