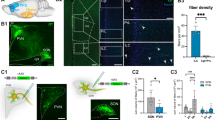
Abstract
Exposure to stress can affect the establishment of dominance hierarchies. In our model, a social hierarchy established by two male rats during a first encounter is not maintained 1 week later. If one of the two rats is stressed, the stressed rat becomes subordinate and the hierarchy that is formed is maintained. In this study, we investigated the changes in the expression of oxytocin (Otr) and vasopressin (V1aR) receptor genes in the medial amygdala (MeA) and the lateral septum (LS) in the hours following hierarchy establishment under both stressed and basal conditions. We found that the potentiation of a social hierarchy induced by stress is accompanied by social status- and region-specific changes in the expression of Otr mRNA in the MeA 3 h after the social encounter. At this time point, no evidence was found for the regulation of V1aR mRNA in any of the brain regions examined. Results from pharmacological experiments involving the microinfusion of a specific OTR antagonist immediately after the acquisition of a subordinate status under basal, non-stress conditions suggested a role for this receptor in the MeA on the long-term establishment of the subordinate status. Altogether, these findings highlight a role for the oxytocinergic system in the mechanisms through which stress facilitates the long-term establishment of a social hierarchy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Benelli A, Bertolini A, Poggioli R, Menozzi B, Basaglia R, Arletti R (1995). Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides 28: 251–255.
Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ (2005). The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron 47: 503–513.
Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ (2004). Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29: 483–493.
Bielsky IF, Young LJ (2004). Oxytocin, vasopressin, and social recognition in mammals. Peptides 25: 1565–1574.
Blanchard RJ, Flannelly KJ, Blanchard DC (1988). Life-span studies of dominance and aggression in established colonies of laboratory rats. Physiol Behav 43: 1–7.
Bluthe RM, Schoenen J, Dantzer R (1990). Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res 519: 150–157.
Bosch O, Sartori SB, Singewald N, Neumann ID (2007). Extracellular amino acid levels in the paraventricular nucleus and the central amygdala in high- and low-anxiety dams rats during maternal aggression: regulation by oxytocin. Stress 10: 261–270.
Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW (2007). Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci USA 104: 4670–4675.
Cordero MI, Sandi C (2007). Stress amplifies memory for social hierarchy. Front Neurosci 1: 175–184.
Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM et al. (2007). Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides 41: 145–163.
Dantzer R, Bluthe RM, Koob GF, Le Moal M (1987). Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology 91: 363–368.
Dantzer R, Koob GF, Bluthe RM, Le Moal M (1988). Septal vasopressin modulates social memory in male rats. Brain Res 457: 143–147.
Delville Y, Mansour KM, Ferris CF (1996). Serotonin blocks vasopressin-facilitated offensive aggression: interactions within the ventrolateral hypothalamus of golden hamsters. Physiol Behav 59: 813–816.
Di Scala-Guenot D, Strosser MT (1995). Downregulation of the oxytocin receptor on cultured astroglial cells. Am J Physiol 268: C413–C418.
Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID (2005). Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology 30: 223–230.
Ebner K, Wotjak CT, Landgraf R, Engelmann M (2000). A single social defeat experience selectively stimulates the release of oxytocin, but not vasopressin, within the septal brain area of male rats. Brain Res 872: 87–92.
Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT (1999). Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J Neuroendocrinol 11: 867–872.
Engelmann M, Ebner K, Wotjak CT, Landgraf R (1998). Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav Brain Res 90: 89–94.
Engelmann M, Landgraf R (1994). Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav 55: 145–149.
Engelmann M, Wotjak CT, Ebner K, Landgraf R (2000). Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp Physiol 85: 125S–130S.
Engelmann M, Wotjak CT, Neumann ID, Ludwig M, Landgraf R (1996). Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci Biobehav Rev 20: 341–358.
Everts HG, Koolhaas JM (1997). Lateral septal vasopressin in rats: role in social and object recognition? Brain Res 760: 1–7.
Everts HG, Koolhaas JM (1999). Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behav Brain Res 99: 7–16.
Ferguson JN, Aldag JM, Insel TR, Young LJ (2001). Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 21: 8278–8285.
Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT (2000). Social amnesia in mice lacking the oxytocin gene. Nat Genet 25: 284–288.
Ferguson JN, Young LJ, Insel TR (2002). The neuroendocrine basis of social recognition. Front Neuroendocrinol 23: 200–224.
Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE (1984). Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science 224: 521–523.
Gimpl G, Fahrenholz F (2001). The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81: 629–683.
Goodson JL (2005). The vertebrate social behavior network: evolutionary themes and variations. Horm Behav 48: 11–22.
Herrero AI, Sandi C, Venero C (2006). Individual differences in anxiety traits are related to spatial learning abilities and hippocampal expression of mineralocorticoid receptors. Neurobiol Learn Mem 86: 150–159.
Insel TR, Winslow JT, Witt DM (1992). Homologous regulation of brain oxytocin receptors. Endocrinology 130: 2602–2608.
Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF (1990). Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol Behav 48: 693–699.
Koolhaas JM, de Boer SF, Coppens CM, Buwalda B (2010). Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol 31: 307–321.
Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X et al. (2003). Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur J Neurosci 18: 403–411.
Landgraf R, Wotjak CT, Neumann ID, Engelmann M (1998). Release of vasopressin within the brain contributes to neuroendocrine and behavioral regulation. Prog Brain Res 119: 201–220.
Le Moal M, Dantzer R, Michaud B, Koob GF (1987). Centrally injected arginine vasopressin (AVP) facilitates social memory in rats. Neurosci Lett 77: 353–359.
Neumann ID (2007). Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans 35: 1252–1257.
Neumann ID, Krömer SA, Toschi N, Ebner K (2000). Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept 96: 31–38.
Newman SW (1999). The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann NY Acad Sci 877: 242–257.
Ostrowski NL (1998). Oxytocin receptor mRNA expression in rat brain: implications for behavioral integration and reproductive success. Psychoneuroendocrinology 23: 989–1004.
Ostrowski NL, Lolait SJ, Young III WS (1994). Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology 135: 1511–1528.
Patchev VK, Schlosser SF, Hassan AH, Almeida OF (1993). Oxytocin binding sites in rat limbic and hypothalamic structures: site-specific modulation by adrenal and gonadal steroids. Neuroscience 57: 537–543.
Popik P, Van Ree JM (1991). Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol 1: 555–560.
Popik P, Van Ree JM (1992). Long-term facilitation of social recognition in rats by vasopressin related peptides: a structure-activity study. Life Sci 50: 567–572.
Popik P, Vetulani J (1991). Opposite action of oxytocin and its peptide antagonists on social memory in rats. Neuropeptides 18: 23–27.
Popik P, Vetulani J, van Ree JM (1992). Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology 106: 71–74.
Popik P, Vetulani J, Van Ree JM (1996). Facilitation and attenuation of social recognition in rats by different oxytocin-related peptides. Eur J Pharmacol 308: 113–116.
Popik P, Wolterink G, De Brabander H, van Ree JM (1991). Neuropeptides related to [Arg8]vasopressin facilitates social recognition in rats. Physiol Behav 49: 1031–1035.
Timmer M, Sandi C (2010). A role for glucocorticoids in the long-term establishment of a social hierarchy. Psychoneuroendocrinology 35: 1543–1552.
Van Kreveld D (1970). A selective review of dominance-subordinate relations in animals. Genet Psychol Monogr 81: 143–173.
van Wimersma Greidanus TB, Maigret C (1996). The role of limbic vasopressin and oxytocin in social recognition. Brain Res 713: 153–159.
Veenema AH, Neumann ID (2008). Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res 170: 261–276.
Watters JJ, Wilkinson CW, Dorsa DM (1996). Glucocorticoid regulation of vasopressin V1a receptors in rat forebrain. Brain Res Mol Brain Res 38: 276–284.
Winslow JT, Insel TR (1993). Effects of central vasopressin administration to infant rats. Eur J Pharmacol 233: 101–107.
Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M (1998). Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience 85: 1209–1222.
Wotjak CT, Kubota M, Liebsch G, Montkowski A, Holsboer F, Neumann ID et al. (1996). Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: a novel mechanism of regulating adrenocorticotropic hormone secretion? J Neurosci 16: 7725–7732.
Acknowledgements
This work was supported by Grants from the EU 7th (FP7-HEALTH-F2M-2007-201600; MemStick) FP, the Swiss National Science Foundation (310000-120791; Sinergia CRSIK0-122697 and CRSIK0-122691) and the Swiss Network for International Studies (SNIS) to CS. We thank Grégoire Parchet and Angélique Vaucher for technical assistance and Prof Ruth Lüthi-Carter, Manuel Bueno, and Sylvain Lengacher for their advice on the gene expression experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Timmer, M., Cordero, M., Sevelinges, Y. et al. Evidence for a Role of Oxytocin Receptors in the Long-Term Establishment of Dominance Hierarchies. Neuropsychopharmacol 36, 2349–2356 (2011). https://doi.org/10.1038/npp.2011.125
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2011.125
Keywords
This article is cited by
-
Novel competition test for food rewards reveals stable dominance status in adult male rats
Scientific Reports (2021)
-
Stress-induced changes in social dominance are scaled by AMPA-type glutamate receptor phosphorylation in the medial prefrontal cortex
Scientific Reports (2018)
-
Neuroligin-2 Expression in the Prefrontal Cortex is Involved in Attention Deficits Induced by Peripubertal Stress
Neuropsychopharmacology (2016)
-
Dominance status predicts social fear transmission in laboratory rats
Animal Cognition (2016)
-
Stress and the social brain: behavioural effects and neurobiological mechanisms
Nature Reviews Neuroscience (2015)