Abstract
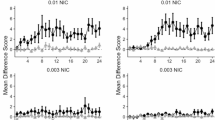
Nicotine has both unconditioned and conditioned stimulus properties. Conditioned stimulus properties of nicotine may contribute to the tenacity of nicotine addiction. The purpose of this experiment was to use neurohistochemical analysis of rapidly developing c-Fos protein to elucidate neurobiological loci involved in the processing of nicotine as an interoceptive conditioned stimulus (CS). Rats were injected (SC) in an intermixed fashion with saline or nicotine (16 sessions of each) and placed in conditioning chambers where they were given one of the three conditions depending on group assignment: (a) nicotine paired 100% of the time with intermittent access to sucrose (nicotine-CS condition), (b) nicotine and saline each paired 50% of the time with sucrose (chamber-CS condition), or (c) no sucrose US control (CS-alone condition). Rats in the nicotine-CS condition acquired the discrimination as evidenced by goal-tracking (ie, increased dipper entries before initial sucrose delivery) only on nicotine sessions. The chamber-CS condition showed goal-tracking on all sessions; no goal-tracking was seen in the CS-alone condition. On the test day, rats in each condition were challenged with saline or nicotine and later assessed for c-Fos immunoreactivity. In concordance with previous reports, nicotine induced c-Fos expression in the majority of areas tested; however, learning-dependent expression was specific to dorsomedial and ventromedial regions of caudate-putamen (dmCPu, vmCPu). Only rats in the nicotine-CS condition, when challenged with nicotine, had higher c-Fos expression in the dmCPu and vmCPu. These results suggest that medial areas of CPu involved in excitatory conditioning with an appetitive nicotine CS.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abramoff MD, Magelhaes PJ, Ram SJ (2004). Image processing with imageJ. Biophoton Int 11: 36–42.
Alessi SM, Roll JM, Reilly MP, Johanson CE (2002). Establishment of a diazepam preference in human volunteers following a differential-conditioning history of placebo versus diazepam choice. Exp Clin Psychopharmacol 10: 77–83, discussion 101–103.
Azizian A, Monterosso J, O’neill J, London ED (2009). Magnetic resonance imaging studies of cigarette smoking. Handb Exp Pharmacol 192: 113–143.
Balfour DJ (2009). The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handb Exp Pharmacol 192: 209–233.
Balleine BW, Dickinson A (1998). Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37: 407–419.
Barik J, Wonnacott S (2009). Molecular and cellular mechanisms of action of nicotine in the Cns. Handb Exp Pharmacol 192: 173–207.
Berke JD, Hyman SE (2000). Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25: 515–532.
Besheer J, Palmatier M, Metschke D, Bevins R (2004). Nicotine as a signal for the presence or absence of sucrose reward: a pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berl) 172: 108–117.
Bevins R, Besheer J, Pickett K (2001). Nicotine-conditioned locomotor activity in rats: dopaminergic and gabaergic influences on conditioned expression. Pharmacol Biochem Behav 68: 135–145.
Bevins RA (2009). Altering the motivational function of nicotine through conditioning processes. Nebr Symp Motiv 55: 111–129.
Bevins RA, Caggiula AR (2009). Nicotine, tobacco use, and the 55th Nebraska symposium on motivation. Nebr Symp Motiv 55: 1–3.
Bevins RA, Murray JE (2011). Internal stimuli generated by abused substances: role of pavlovian conditioning and its implications for drug addiction. In: Schachtman T, Reilly S (Eds). Associative Learning and Conditioning: Human and Non-Human Applications. Oxford University Press: New York, Ny. pp 270–289.
Bevins RA, Palmatier MI (2004). Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev 3: 143–158.
Bevins RA, Penrod RD, Reichel CM (2007). Nicotine does not produce state-dependent effects on learning in a pavlovian appetitive goal tracking task with rats. Behav Brain Res 177: 134–141.
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA et al (2001). Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70: 515–530.
CDC (2008). Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. Morbidity and Mortality Weekly Report Vol 2010.
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L et al (2006). Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 189: 27–36.
Conklin CA, Tiffany ST (2002). Cue-exposure treatment: time for change. Addiction 97: 1219–1221.
Corrigall W (1999). Nicotine self-administration in animals as a dependence model. Nicotine Tob Res 1: 11–20.
Corrigall W, Coen K (1989). Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 99: 473–478.
Corrigall W, Coen K, Adamson K (1994). Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653: 278–284.
Corrigall W, Franklin K, Coen K, Clarke P (1992). The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 107: 285–289.
Coutureau E, Killcross S (2003). Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in overtrained rats. Behav Brain Res 146: 167–174.
Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ (1995). Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64: 477–505.
Curran T, Morgan JI (1995). Fos: an immediate-early transcription factor in neurons. J Neurobiol 26: 403–412.
Dalley JW, Cardinal RN, Robbins TW (2004). Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28: 771–784.
Di Chiara G (2000). Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol 393: 295–314.
Everitt BJ, Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481–1489.
Everitt BJ, Wolf ME (2002). Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 22: 3312–3320.
Farwell BJ, Ayres JJB (1979). Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (‘Goal Tracking’) in rats. Learn Motiv 10: 295–312.
Institute Of Laboratory Animal Resources (U.S.) (1996). Guide for the Care and Use of Laboratory Animals, 7th edn. National Academy Press: Washington, D.C., xii, 125 P.Pp.
Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ (2000). Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci 20: 7489–7495.
Ito R, Dalley JW, Robbins TW, Everitt BJ (2002). Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci 22: 6247–6253.
Killcross S, Coutureau E (2003). Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex 13: 400–408.
Kovács KJ (1998). c-Fos as a transcription factor: a stressful (Re)view from a functional map. Neurochem Int 33: 287–297.
Marttila K, Raattamaa H, Ahtee L (2006). Effects of chronic nicotine administration and its withdrawal on striatal Fosb/Deltafosb and c-Fos expression in rats and mice. Neuropharmacology 51: 44–51.
Murray JE, Bevins RA (2007a). Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol 561: 91–104.
Murray JE, Bevins RA (2007b). The conditional stimulus effects of nicotine vary as a function of training dose. Behav Pharmacol 18: 707–716.
Murray JE, Bevins RA (2011). Excitatory conditioning to the interoceptive nicotine stimulus blocks subsequent conditioning to an exteroceptive light stimulus. Behav Brain Res 221: 314–319.
Murray JE, Penrod RD, Bevins RA (2009). Nicotine-evoked conditioned responding is dependent on concentration of sucrose unconditioned stimulus. Behav Process 81: 136–139.
Murray JE, Walker AW, Polewan RJ, Bevins RA (2011). An examination of nmda receptor contribution to conditioned responding evoked by the conditional stimulus effects of nicotine. Psychopharmacology (Berl) 213: 131–141.
Neisewander JL, O’dell LE, Tran-Nguyen LT, Castañeda E, Fuchs RA (1996). Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology 15: 506–514.
Olausson P, Jentsch JD, Taylor JR (2004). Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 171: 173–178.
Pagliusi SR, Tessari M, Devevey S, Chiamulera C, Pich EM (1996). The reinforcing properties of nicotine are associated with a specific patterning of c-Fos expression in the rat brain. Eur J Neurosci 8: 2247–2256.
Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA (2005). Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a pavlovian appetitive discrimination task in rats. Neuropsychopharmacology 30: 731–741.
Paxinos G, Watson C (2007). The Rat Brain in Stereotaxic Coordinates, 6th edn. Academic Press: San Diego, CA, USA.
Rhodes JS, Ryabinin AE, Crabbe JC (2005). Patterns of brain activation associated with contextual conditioning to methamphetamine in mice. Behav Neurosci 119: 759–771.
Robbins TW (2005). Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol 493: 140–146.
Robinson TE, Berridge KC (2003). Addiction. Annu Rev Psychol 54: 25–53.
Salminen O, Seppä T, Gäddnäs H, Ahtee L (1999). The effects of acute nicotine on the metabolism of dopamine and the expression of fos protein in striatal and limbic brain areas of rats during chronic nicotine infusion and its withdrawal. J Neurosci 19: 8145–8151.
Schultz W (1998). Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27.
Schultz W (2006). Behavioral theories and the neurophysiology of reward. Annu Rev Psychol 57: 87–115.
Senba E, Ueyama T (1997). Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci Res 29: 183–207.
Shram MJ, Funk D, Li Z, Lê AD (2007). Acute nicotine enhances c-Fos Mrna expression differentially in reward-related substrates of adolescent and adult rat brain. Neurosci Lett 418: 286–291.
Smith KS, Tindell AJ, Aldridge JW, Berridge KC (2009). Ventral pallidum roles in reward and motivation. Behav Brain Res 196: 155–167.
Squire LR (1992). Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99: 195–231.
Struthers AM, Wilkinson JL, Dwoskin LP, Crooks PA, Bevins RA (2009). Mecamylamine, dihydro-beta-erythroidine, and dextromethorphan block conditioned responding evoked by the conditional stimulus effects of nicotine. Pharmacol Biochem Behav 94: 319–328.
Tiffany ST (1990). A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev 97: 147–168.
Vanderschuren LJ, Di Ciano P, Everitt BJ (2005a). Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci 25: 8665–8670.
Vanderschuren LJ, Everitt BJ (2005b). Behavioral and neural mechanisms of compulsive drug seeking. Eur J Pharmacol 526: 77–88.
Zhao C, Li M (2010). c-Fos identification of neuroanatomical sites associated with haloperidol and clozapine disruption of maternal behavior in the rat. Neuroscience 166: 1043–1055.
Acknowledgements
This work was supported by NIH research grant DA018114 and DA023951. We thank Catalin V. Buhusi for his thoughtful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Charntikov, S., Tracy, M., Zhao, C. et al. Conditioned Response Evoked by Nicotine Conditioned Stimulus Preferentially Induces c-Fos Expression in Medial Regions of Caudate-Putamen. Neuropsychopharmacol 37, 876–884 (2012). https://doi.org/10.1038/npp.2011.263
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2011.263
Keywords
This article is cited by
-
Behavioral and Neural Substrates of Habit Formation in Rats Intravenously Self-Administering Nicotine
Neuropsychopharmacology (2014)