Abstract
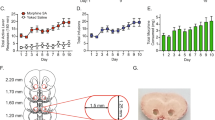
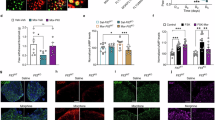
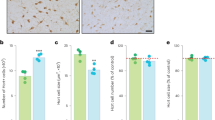
It has been established that mu opioid receptors activate the ERK1/2 signaling cascade both in vitro and in vivo. The Ser/Thr kinase RSK2 is a direct downstream effector of ERK1/2 and has a role in cellular signaling, cell survival growth, and differentiation; however, its role in biological processes in vivo is less well known. Here we determined whether RSK2 contributes to mu-mediated signaling in vivo. Knockout mice for the rsk2 gene were tested for main morphine effects, including analgesia, tolerance to analgesia, locomotor activation, and sensitization to this effect, as well as morphine withdrawal. The deletion of RSK2 reduced acute morphine analgesia in the tail immersion test, indicating a role for this kinase in mu receptor-mediated nociceptive processing. All other morphine effects and adaptations to chronic morphine were unchanged. Because the mu opioid receptor and RSK2 both show high density in the habenula, we specifically downregulated RSK2 in this brain metastructure using an adeno-associated-virally mediated shRNA approach. Remarkably, morphine analgesia was significantly reduced, as observed in the total knockout animals. Together, these data indicate that RSK2 has a role in nociception, and strongly suggest that a mu opioid receptor–RSK2 signaling mechanism contributes to morphine analgesia at the level of habenula. This study opens novel perspectives for both our understanding of opioid analgesia, and the identification of signaling pathways operating in the habenular complex.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Accession codes
References
Anjum R, Blenis J (2008). The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol 9: 747–758.
Basbaum AI, Bautista DM, Scherrer G, Julius D (2009). Cellular and molecular mechanisms of pain. Cell 139: 267–284.
Brami-Cherrier K, Roze E, Girault JA, Betuing S, Caboche J (2009). Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. J Neurochem 108: 1323–1335.
Cao JL, He JH, Ding HL, Zeng YM (2005). Activation of the spinal ERK signaling pathway contributes naloxone-precipitated withdrawal in morphine-dependent rats. Pain 118: 336–349.
Chotteau-Lelievre A, Dolle P, Gofflot F (2006). Expression analysis of murine genes using in situ hybridization with radioactive and nonradioactively labeled RNA probes. Methods Mol Biol 326: 61–87.
Cohen SR, Melzack R (1985). Morphine injected into the habenula and dorsal posteromedial thalamus produces analgesia in the formalin test. Brain Res 359: 131–139.
Cohen SR, Melzack R (1986). Habenular stimulation produces analgesia in the formalin test. Neurosci Lett 70: 165–169.
Contet C, Filliol D, Matifas A, Kieffer BL (2008). Morphine-induced analgesic tolerance, locomotor sensitization and physical dependence do not require modification of mu opioid receptor, cdk5 and adenylate cyclase activity. Neuropharmacology 54: 475–486.
Contet C, Kieffer BL, Befort K (2004). Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol 14: 370–378.
Darcq E, Koebel P, Del Boca C, Pannetier S, Kirstetter AS, Garnier JM et al (2011). RSK2 signaling in brain habenula contributes to place aversion learning. Learn Mem 18: 574–578.
Dickenson AH, Kieffer B (2006). Opiates: Basic Mechanisms. Elsevier: London Churchill Livingstone: NY.
Dufresne SD, Bjorbaek C, El-Haschimi K, Zhao Y, Aschenbach WG, Moller DE et al (2001). Altered extracellular signal-regulated kinase signaling and glycogen metabolism in skeletal muscle from p90 ribosomal S6 kinase 2 knockout mice. Mol Cell Biol 21: 81–87.
Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith Jr D et al (2003). Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J Neurosci 23: 8360–8369.
El-Haschimi K, Dufresne SD, Hirshman MF, Flier JS, Goodyear LJ, Bjorbaek C (2003). Insulin resistance and lipodystrophy in mice lacking ribosomal S6 kinase 2. Diabetes 52: 1340–1346.
Fields H (2004). State-dependent opioid control of pain. Nat Rev Neurosci 5: 565–575.
Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA (2005). Mechanisms for selection of basic motor programs—roles for the striatum and pallidum. Trends Neurosci 28: 364–370.
Hanauer A, Young ID (2002). Coffin-Lowry syndrome: clinical and molecular features. J Med Genet 39: 705–713.
Hashimoto K, Amano T, Sakai N, Suzuki T, Narita M (2009). Cell-dependent physiological synaptic action of morphine in the rat habenular nucleus: morphine both inhibits and facilitates excitatory synaptic transmission. Neurosci Lett 451: 270–273.
Hikosaka O (2010). The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci 11: 503–513.
Kieffer BL, Gaveriaux-Ruff C (2002). Exploring the opioid system by gene knockout. Prog Neurobiol 66: 285–306.
Kitchen I, Slowe SJ, Matthes HW, Kieffer B (1997). Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Res 778: 73–88.
Le Merrer J, Becker JA, Befort K, Kieffer BL (2009). Reward processing by the opioid system in the brain. Physiol Rev 89: 1379–1412.
Le Merrer J, Befort K, Gardon O, Filliol D, Darcq E, Dembele D et al (2011). Protracted abstinence from drugs of abuse shows common gene network regulations. Addiction Biology (28 September 2011, doi:10.1111/j.1369-1600.2011.00365.x (e-pub ahead of print)).
Lecourtier L, Kelly PH (2007). A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev 31: 658–672.
Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM et al (2008). Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci 28: 13248–13257.
Mahieux G, Benabid AL (1987). Naloxone-reversible analgesia induced by electrical stimulation of the habenula in the rat. Brain Res 406: 118–129.
Mansour A, Fox CA, Akil H, Watson SJ (1995). Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 18: 22–29.
Marques Pereira P, Gruss M, Braun K, Foos N, Pannetier S, Hanauer A (2008). Dopaminergic system dysregulation in the mrsk2_KO mouse, an animal model of the Coffin-Lowry syndrome. J Neurochem 107: 1325–1334.
Narita M, Hashimoto K, Amano T, Narita M, Niikura K, Nakamura A et al (2008). Post-synaptic action of morphine on glutamatergic neuronal transmission related to the descending antinociceptive pathway in the rat thalamus. J Neurochem 104: 469–478.
Ozaki S, Narita M, Narita M, Ozaki M, Khotib J, Suzuki T (2004). Role of extracellular signal-regulated kinase in the ventral tegmental area in the suppression of the morphine-induced rewarding effect in mice with sciatic nerve ligation. J Neurochem 88: 1389–1397.
Paxinos G, Franklin K (2001). The Mouse Brain in Stereotaxic Coordinates. Academic Press: San Diego.
Pereira PM, Schneider A, Pannetier S, Heron D, Hanauer A (2010). Coffin-Lowry syndrome. Eur J Hum Genet 18: 627–633.
Poirier R, Jacquot S, Vaillend C, Soutthiphong AA, Libbey M, Davis S et al (2007). Deletion of the Coffin-Lowry syndrome gene Rsk2 in mice is associated with impaired spatial learning and reduced control of exploratory behavior. Behav Genet 37: 31–50.
Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL (2004). The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci 19: 2239–2248.
Temtamy SA, Miller JD, Hussels-Maumenee I (1975). The Coffin-Lowry syndrome: an inherited faciodigital mental retardation syndrome. J Pediatr 86: 724–731.
Terenzi MG, Guimaraes FS, Prado WA (1990). Antinociception induced by stimulation of the habenular complex of the rat. Brain Res 524: 213–218.
Terenzi MG, Prado WA (1990). Antinociception elicited by electrical or chemical stimulation of the rat habenular complex and its sensitivity to systemic antagonists. Brain Res 535: 18–24.
Thornton EW, Evans JA (1984). The effects of lesions of the habenula nucleus on lever press behaviour during a tandem operant schedule with contrasting response requirements. Behav Brain Res 12: 327–334.
Trivier E, De Cesare D, Jacquot S, Pannetier S, Zackai E, Young I et al (1996). Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature 384: 567–570.
Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D (2006). Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci 7: 20.
Valjent E, Pages C, Herve D, Girault JA, Caboche J (2004). Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci 19: 1826–1836.
Williams JT, Christie MJ, Manzoni O (2001). Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81: 299–343.
Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T et al (2004). ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell 117: 387–398.
Zeniou M, Ding T, Trivier E, Hanauer A (2002). Expression analysis of RSK gene family members: the RSK2 gene, mutated in Coffin-Lowry syndrome, is prominently expressed in brain structures essential for cognitive function and learning. Hum Mol Genet 11: 2929–2940.
Acknowledgements
We thank O Gardon for cloning and supplying the oprm1 probe, J-M Garnier for AAV2-shRNA construction, A-S Kirstetter for technical assistance with stereotaxic experiments, JA Becker for helpful comments and advice. We thank G Duval and D Memedov for animal care. This work was supported by the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, and Université de Strasbourg. We thank the European Union (GENADDICT/FP6 005166), and the National Institutes of Health (NIAAA AA-16658 and NIDA DA-16768), for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Darcq, E., Befort, K., Koebel, P. et al. RSK2 Signaling in Medial Habenula Contributes to Acute Morphine Analgesia. Neuropsychopharmacol 37, 1288–1296 (2012). https://doi.org/10.1038/npp.2011.316
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2011.316
Keywords
This article is cited by
-
Neuropeptide Y in the medial habenula alleviates migraine-like behaviors through the Y1 receptor
The Journal of Headache and Pain (2023)
-
Regulation of habenular G-protein gamma 8 on learning and memory via modulation of the central acetylcholine system
Molecular Psychiatry (2021)
-
Mu opioid receptors in the medial habenula contribute to naloxone aversion
Neuropsychopharmacology (2020)