Abstract
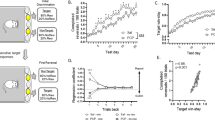
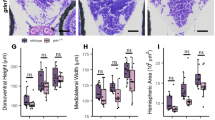
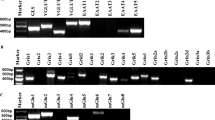
N-methyl-D-aspartate (NMDA) receptor is a glutamate receptor which has an important role on mammalian brain development. We have reported that prenatal treatment with phencyclidine (PCP), a NMDA receptor antagonist, induces long-lasting behavioral deficits and neurochemical changes. However, the mechanism by which the prenatal antagonism of NMDA receptor affects neurodevelopment, resulting in behavioral deficits, has remained unclear. Here, we report that prenatal NMDA receptor antagonism impaired the proliferation of neuronal progenitors, leading to a decrease in the progenitor pool in the ventricular and the subventricular zone. Furthermore, using a PCR array focused on neurogenesis and neuronal stem cells, we evaluated changes in gene expression causing the impairment of neuronal progenitor proliferation and found aberrant gene expression, such as Notch2 and Ntn1, in prenatal PCP-treated mice. Consequently, the density of glutamatergic neurons in the prefrontal cortex was decreased, probably resulting in glutamatergic hypofunction. Prenatal PCP-treated mice displayed behavioral deficits in cognitive memory and sensorimotor gating until adulthood. These findings suggest that NMDA receptors regulate the proliferation and maturation of progenitor cells for glutamatergic neuron during neurodevelopment, probably via the regulation of gene expression.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abekawa T, Ito K, Nakagawa S, Koyama T (2007). Prenatal exposure to an NMDA receptor antagonist, MK-801 reduces density of parvalbumin-immunoreactive GABAergic neurons in the medial prefrontal cortex and enhances phencyclidine-induced hyperlocomotion but not behavioral sensitization to methamphetamine in postpubertal rats. Psychopharmacology (Berlin) 192: 303–316.
Asahi M, Hoshimaru M, Hojo M, Matsuura N, Kikuchi H, Hashimoto N (1998). Induction of the N-methyl-D-aspartate receptor subunit 1 in the immortalized neuronal progenitor cell line HC2S2 during differentiation into neurons. J Neurosci Res 52: 699–708.
Brazel CY, Nuñez JL, Yang Z, Levison SW (2005). Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience 131: 55–65.
Cirulli V, Yebra M (2007). Netrins: beyond the brain. Nat Rev Mol Cell Biol 8: 296–306.
Dehay C, Kennedy H (2007). Cell-cycle control and cortical development. Nat Rev Neurosci 8: 438–450.
Fico TA, Vanderwende C (1988). Phencyclidine during pregnancy: fetal brain levels and neurobehavioral effects. Neurotoxicol Teratol 10: 349–354.
Fitzgerald DP, Bradford D, Cooper HM (2007). Neogenin is expressed on neurogenic and gliogenic progenitors in the embryonic and adult central nervous system. Gene Expr Patterns 7: 784–792.
Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M et al (1994). Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron 13: 325–338.
Götz M, Huttner WB (2005). The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6: 777–788.
Hamada Y, Kadokawa Y, Okabe M, Ikawa M, Coleman JR, Tsujimoto Y (1999). Mutation in ankyrin repeats of the mouse Notch2 gene induces early embryonic lethality. Development 126: 3415–3424.
Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA et al (2002). Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci 5: 308–315.
Hevner RF (2006). From radial glia to pyramidal-projection neuron: transcription factor cascades in cerebral cortex development. Mol Neurobiol 33: 33–50.
Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vöckler J, Dikranian K et al (1999). Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283: 70–74.
Joo JY, Kim BW, Lee JS, Park JY, Kim S, Yun YJ et al (2007). Activation of NMDA receptors increases proliferation and differentiation of hippocampal neural progenitor cells. J Cell Sci 120: 1358–1370.
Kaneko T, Mizuno N (1994). Glutamate-synthesizing enzymes in GABAergic neurons of the neocortex: a double immunofluorescence study in the rat. Neuroscience 61: 839–849.
Kaufman KR, Petrucha RA, Pitts Jr FN, Weekes ME (1983). PCP in amniotic fluid and breast milk: case report. J Clin Psychiatry 44: 269–270.
Komuro H, Rakic P (1993). Modulation of neuronal migration by NMDA receptors. Science 260: 95–97.
Koseki T, Mouri A, Mamiya T, Aoyama Y, Toriumi K, Suzuki S et al (2011). Exposure to enriched environments during adolescence prevents abnormal behaviors associated with histone deacetylation in phencyclidine-treated mice. Int J Neuropsychopharmacol (in press).
Lewis DA, Glantz LA, Pierri JN, Sweet RA (2003). Altered cortical glutamate neurotransmission in schizophrenia: evidence from morphological studies of pyramidal neurons. Ann NY Acad Sci 1003: 102–112.
Llambi F, Causeret F, Bloch-Gallego E, Mehlen P (2001). Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J 20: 2715–2722.
López T, López-Colomé AM, Ortega A (1997). NMDA receptors in cultured radial glia. FEBS Lett 405: 245–248.
Louvi A, Artavanis-Tsakonas S (2006). Notch signalling in vertebrate neural development. Nat Rev Neurosci 7: 93–102.
Lu L, Mamiya T, Lu P, Toriumi K, Mouri A, Hiramatsu M et al (2010). Prenatal exposure to phencyclidine produces abnormal behavior and NMDA receptor expression in postpubertal mice. Int J Neuropsychopharmacol 13: 877–889.
Lu L, Mamiya T, Lu P, Toriumi K, Mouri A, Hiramatsu M et al (2011). Prenatal exposure to PCP produces behavioral deficits accompanied by the overexpression of GLAST in the prefrontal cortex of postpubertal mice. Behav Brain Res 220: 132–139.
Luk KC, Kennedy TE, Sadikot AF (2003). Glutamate promotes proliferation of striatal neuronal progenitors by an NMDA receptor-mediated mechanism. J Neurosci 23: 2239–2250.
Maddox VH, Godefroi EF, Parcell RF (1965). The synthesis of phencyclidine and other 1-arylcyclohexylamines. J Med Chem 8: 230–235.
McConnell SK (1995). Constructing the cerebral cortex: neurogenesis and fate determination. Neuron 15: 761–768.
Mochizuki N, Takagi N, Kurokawa K, Kawai T, Besshoh S, Tanonaka K et al (2007). Effect of NMDA receptor antagonist on proliferation of neurospheres from embryonic brain. Neurosci Lett 417: 143–148.
Mouri A, Noda Y, Hara H, Mizoguchi H, Tabira T, Nabeshima T (2007). Oral vaccination with a viral vector containing Abeta cDNA attenuates age-related Abeta accumulation and memory deficits without causing inflammation in a mouse Alzheimer model. FASEB J 21: 2135–2148.
Murai R, Noda Y, Matsui K, Kamei H, Mouri A, Matsuba K et al (2007). Hypofunctional glutamatergic neurotransmission in the prefrontal cortex is involved in the emotional deficit induced by repeated treatment with phencyclidine in mice: implications for abnormalities of glutamate release and NMDA-CaMKII signaling. Behav Brain Res 180: 152–160.
Nicholas JM, Lipshitz J, Schreiber EC (1982). Phencyclidine: its transfer across the placenta as well as into breast milk. Am J Obstet Gynecol 143: 143–146.
Pfaffl MW (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45.
Rakic P (1995). A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci 18: 383–388.
Sah DW, Ray J, Gage FH (1997). Regulation of voltage- and ligand-gated currents in rat hippocampal progenitor cells in vitro. J Neurobiol 32: 95–110.
Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME (2001). Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron 31: 557–568.
Suzuki M, Nelson AD, Eickstaedt JB, Wallace K, Wright LS, Svendsen CN (2006). Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci 24: 645–653.
Wang C, McInnis J, Ross-Sanchez M, Shinnick-Gallagher P, Wiley JL, Johnson KM (2001). Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: implications for schizophrenia. Neuroscience 107: 535–550.
Acknowledgements
We thank Dr Furukawa H for synthesizing PCP. This study was supported by Grants-in-aid for Scientific Research (A) (22248033), Scientific Research (B) (20390073) (21390045) and Exploratory Research from the JSPS (19659017) (22659213); by the ‘Academic Frontier’ Project for Private Universities (2007–2011) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT); by Regional Joint Research Program supported by grants to Private Universities to Cover Current Expenses from MEXT; by Research on Regulatory Science of Pharmaceuticals and Medical Devices from the Ministry of Health, Labour and Welfare, Japan (MHLW); by Research on Risk of Chemical Substances, Health and Labour Science Research Grants supported by MHLW; and by the joint research project under the Japan-Korea basic scientific cooperation program (JSPS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Toriumi, K., Mouri, A., Narusawa, S. et al. Prenatal NMDA Receptor Antagonism Impaired Proliferation of Neuronal Progenitor, Leading to Fewer Glutamatergic Neurons in the Prefrontal Cortex. Neuropsychopharmacol 37, 1387–1396 (2012). https://doi.org/10.1038/npp.2011.324
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2011.324
Keywords
This article is cited by
-
Vitamin B6 deficiency hyperactivates the noradrenergic system, leading to social deficits and cognitive impairment
Translational Psychiatry (2021)
-
Protective Potential of the Glutathione Peroxidase-1 Gene in Abnormal Behaviors Induced by Phencyclidine in Mice
Molecular Neurobiology (2017)
-
Adolescent stress leads to glutamatergic disturbance through dopaminergic abnormalities in the prefrontal cortex of genetically vulnerable mice
Psychopharmacology (2017)
-
Prenatal Nicotine Exposure Impairs the Proliferation of Neuronal Progenitors, Leading to Fewer Glutamatergic Neurons in the Medial Prefrontal Cortex
Neuropsychopharmacology (2016)
-
Prenatal phencyclidine treatment induces behavioral deficits through impairment of GABAergic interneurons in the prefrontal cortex
Psychopharmacology (2016)