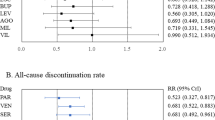
Abstract
Variability in placebo response greatly complicates the design, conduct, and interpretation of clinical trials of antidepressant medications. To identify factors that impact detection of antidepressant–placebo differences, we conducted a meta-analysis of all relevant phase II–IV clinical trials for major depressive disorder conducted by the manufacturer of venlafaxine and desvenlafaxine completed by March 2011. We examined 15 factors potentially relevant to trial outcomes, using the standardized mean difference on the Hamilton Rating Scale for Depression (HAM-D17) score as the primary outcome. Thirty trials comprising 8933 patients were included. In univariate analyses, antidepressant efficacy (ie, drug vs placebo difference) was predicted most strongly (β=3.74, p=0.0002) by the proportion of patients in the trial enrolled from academic sites. Other factors predicting larger drug–placebo differences included lower participant completion rate, fewer post-baseline study visits, earlier year of study, and study drug (venlafaxine>desvenlafaxine). In multivariate meta-regression modeling, only the proportion of patients from academic sites maintained statistical significance as a predictor of drug–placebo separation for both HAM-D17 continuous score change (β=2.24, p=0.034) and response rate (β=2.26, p=0.035). Including a higher proportion of academic sites may increase the ability to detect differences between active drug and placebo in clinical trials of major depressive disorder.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
American Psychiatric Work Group on Major Depressive Disorder (2010). Practice guideline for the treatment of patients with major depressive disorder, 3rd Edition. Available at http://www.psychiatryonline.org/content.aspx?bookid=28§ionid=1667485.
Fava M, Evins AE, Dorer DJ, Schoenfeld DA (2003). The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom 72: 115–127.
Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC et al (2010). Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 303: 47–53.
Frank JD, Frank JB (1991) Persuasion and Healing: A Comparative Study of Psychotherapy. Johns Hopkins Press, Baltimore, MD.
Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62.
Higgins JP, Thompson SG (2002). Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558.
Higgins JPT, Green S (eds) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration (2011). Available from www.cochrane-handbook.org.
Hrobjartsson A, Gotzsche PC (2001). Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med 344: 1594–1602.
Khan A, Dager SR, Cohen S, Avery DH, Scherzo B, Dunner DL (1991). Chronicity of depressive episode in relation to antidepressant-placebo response. Neuropsychopharmacology 4: 125–130.
Khan A, Leventhal RM, Khan SR, Brown WA (2002). Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol 22: 40–45.
Khan A, Schwartz K, Kolts RL, Ridgway D, Lineberry C (2007). Relationship between depression severity entry criteria and antidepressant clinical trial outcomes. Biol Psychiatry 62: 65–71.
Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT (2008). Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 5: e45.
Kobak KA, Brown B, Sharp I, Levy-Mack H, Wells K, Ockun F et al (2009). Sources of unreliability in depression ratings. J Clin Psychopharmacol 29: 82–85.
Kraemer HC, Wilson GT, Fairburn CG, Agras WS (2002). Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 59: 877–883.
Landin R, DeBrota DJ, DeVries TA, Potter WZ, Demitrack MA (2000). The impact of restrictive entry criterion during the placebo lead-in period. Biometrics 56: 271–278.
National Collaborating Centre for Mental Health (2004). Depression: Management of depression in primary and secondary care. Clinical guideline 23. Available at http://www.nice.org.uk/CG23.
Posternak MA, Zimmerman M (2007). Therapeutic effect of follow-up assessments on antidepressant and placebo response rates in antidepressant efficacy trials: meta-analysis. Br J Psychiatry 190: 287–292.
Rettig RA (2000). The industrialization of clinical research. Health Aff 19: 129–146.
Rief W, Nestoriuc Y, Weiss S, Welzel E, Barsky AJ, Hofmann SG (2009). Meta-analysis of the placebo response in antidepressant trials. J Affect Disord 118: 1–8.
Rutherford BR, Wager TD, Roose SP (2010). Expectancy and the treatment of depression: a review of experimental methodology and effects on patient outcome. Curr Psychiatry Rev 6: 1–10.
Sinyor M, Levitt AJ, Cheung AH, Schaffer A, Kiss A, Dowlati Y et al (2010). Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. J Clin Psychiatry 71: 270–279.
Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R (2008). Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med 358: 252–260.
Walsh BT, Seidman SN, Sysko R, Gould M (2002). Placebo response in studies of major depression: variable, substantial, and growing. JAMA 287: 1840–1847.
Acknowledgements
This analysis was sponsored by Wyeth Research, which was acquired by Pfizer in October 2009. Dr Dunlop is supported by NIH grant K23MH086690. The corresponding author had full access to all the data in the study, and had final responsibility for the decision to submit for publication. Pfizer provided no compensation to Drs Dunlop or Thase for conducting this analysis. Medical writing support was provided by Peter Mathisen, PhD and Dennis Stancavish, MA of Embryon LLC, A Division of Advanced Health Media, and Diane Sloan, PharmD and Lorraine Sweeney, BA of Peloton Advantage, LLC and was funded by Pfizer.
Author contributions
Drs Dunlop and Thase were responsible for the design of the analysis. Mr Musgnung, Dr Guico-Pabia, and Dr Ninan reviewed the Pfizer database for the relevant studies and data extraction. Drs Wun and Fayyad jointly conducted the statistical analyses and wrote the statistical analysis section of the Patients and Methods. The remainder of the manuscript was written by Drs Dunlop and Thase. All authors reviewed and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
In the past 3 years, Dr Dunlop has received honoraria for consulting work done for Bristol- Myers Squibb, MedAvante, Inc., and Pfizer. He has also received research support from AstraZeneca, Bristol-Myers Squibb, Evotec, Forest, GlaxoSmithKline, Novartis, Ono Pharmaceuticals, Pfizer, Takeda, and Transcept. Dr Wun was a paid contractor to Pfizer in the development of this manuscript and was responsible for the statistical analysis. Dr Thase has been a consultant/advisor to the following organizations: Alkermes, AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Mylan Laboratories (DeyPharma), Forest Laboratories (PGx), Gerson Lehman Group, Guidepoint Global, H. Lundbeck A/S, MedAvante, Inc., Merck and Co. Inc. (formerly Schering Plough), Neuronetics, Inc., Otsuka, Ortho-McNeil Pharmaceuticals (Johnson & Johnson), Pamlab, L.L.C., Pfizer, PharmaNeuroboost, Shire US Inc., Supernus Pharmaceuticals, Takeda, and Transcept Pharmaceuticals. Dr Thase has also received grant support from the Agency for Healthcare Research and Quality, Eli Lilly &Co., Forest Pharmaceuticals, GlaxoSmithKline (ended July 2010), National Institute of Mental Health, Otsuka Pharmaceuticals, PharmNeuroboost, and Roche. Prior to 30 June 2010, he participated in Speaker Bureaus for the following organizations: AstraZeneca, Bristol-Myers Squibb, Dey Pharmaceutical, Eli Lilly & Co., Merck and Co. Inc., and Pfizer. He has equity holdings in MedAvante, Inc. Dr Thase receives royalties from the American Psychiatric Foundation, Guilford Publications, Herald House and W.W. Norton & Company. His spouse was currently employed with Peloton Advantage, LLC, a company that does business with Pfizer. Drs Guico-Pabia, Fayyad, and Mr Musgnung have been employees of Pfizer for the past 3 years. Dr Ninan was employed by Pfizer during the time this manuscript was developed.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Dunlop, B., Thase, M., Wun, CC. et al. A Meta-analysis of Factors Impacting Detection of Antidepressant Efficacy in Clinical Trials: The Importance of Academic Sites. Neuropsychopharmacol 37, 2830–2836 (2012). https://doi.org/10.1038/npp.2012.153
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2012.153
Keywords
This article is cited by
-
How should we design future mechanistic and/or efficacy clinical trials?
Neuropsychopharmacology (2024)
-
Factors Related to Placebo Response in Randomized, Double-Blind Clinical Trials of Antidepressants in Children and Adolescents: A Meta-regression Analysis
Clinical Drug Investigation (2023)
-
Large multicenter randomized trials in autism: key insights gained from the balovaptan clinical development program
Molecular Autism (2022)
-
Impact of midazolam vs. saline on effect size estimates in controlled trials of ketamine as a rapid-acting antidepressant
Neuropsychopharmacology (2019)
-
Strong placebo response thwarts painkiller trials
Nature (2015)