Abstract
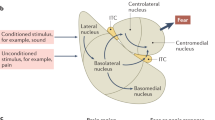
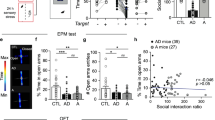
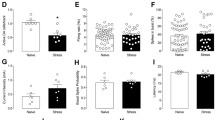
Post-traumatic stress disorder (PTSD) is an anxiety disorder of considerable prevalence in individuals who have experienced a traumatic event. Studies of the neural substrate of this disorder have focused on the role of areas such as the hippocampus, the amygdala and the medial prefrontal cortex. We show that the ventral tegmental area (VTA), which directly modulates these areas, is part of this circuitry. Using a rat model of PTSD, we show that a brief but intense foot shock followed by three brief reminders can cause long-term behavioral changes as shown by anxiety-like, nociception, and touch-sensitivity tests. We show that an intraperitoneal injection of a dopamine (DA) antagonist or a bilateral inactivation of the VTA administered immediately before the traumatic event decrease the occurrence or intensity of these behavioral changes. Furthermore, we show that there is a significant decrease of baseline VTA dopaminergic but not GABAergic cell firing rates 2 weeks after trauma. Our data suggest that VTA DA neurons undergo long-term physiological changes after trauma and that this brain area is a crucial part of the circuits involved in PTSD symptomatology.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anstrom KK, Miczek KA, Budygin EA (2009). Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience 161: 3–12.
Anstrom KK, Woodward DJ (2005). Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology 30: 1832–1840.
Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R et al (2007). Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology 32: 1011–1020.
Asmundson GJ, Coons MJ, Taylor S, Katz J (2002). PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Can J Psychiatry 47: 930–937.
Asmundson GJ, Katz J (2009). Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety 26: 888–901.
Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D (2001). Reward circuitry activation by noxious thermal stimuli. Neuron 32: 927–946.
Borsook D, Becerra L, Carlezon WA, Shaw M, Renshaw P, Elman I et al (2007). Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain 11: 7–20.
Brischoux F, Chakraborty S, Brierley DI, Ungless MA (2009). Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA 106: 4894–4899.
Cao JL, Covington HE, Friedman AK, Wilkinson MB, Walsh JJ, Cooper DC et al (2010). Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci 30: 16453–16458.
Chaouloff F, Durand M, Mormede P (1997). Anxiety–and activity-related effects of diazepam and chlordiazepoxide in the rat light/dark and dark/light tests. Behav Brain Res 85: 27–35.
Cooper DC (2002). The significance of action potential bursting in the brain reward circuit. Neurochem Int 41: 333–340.
Corral-Frías N, Brookshire S, Edelman-Vogelsang K, Valdés J, Fellous J, French E (2010). Efectos del estrés traumático en ratas: estudios conductuales, farmacológicos y electrofisiológicos Vigésimo Congreso de la Sociedad Mexicana del Análisis de la Conducta: Oaxtepec, Morelos, México.
Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM (1989). Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav 32: 777–785.
Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK (2009). Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry 66: 1083–1090.
Ennaceur A, Michalikova S, Chazot PL (2006). Models of anxiety: responses of rats to novelty in an open space and an enclosed space. Behav Brain Res 171: 26–49.
Fields HL, Hjelmstad GO, Margolis EB, Nicola SM (2007). Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci 30: 289–316.
Frayne SM, Chiu VY, Iqbal S, Berg EA, Laungani KJ, Cronkite RC et al (2011). Medical care needs of returning veterans with PTSD: their other burden. J Gen Intern Med 26: 33–39.
Frey U, Schroeder H, Matthies H (1990). Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res 522: 69–75.
Garcia R, Vouimba RM, Baudry M, Thompson RF (1999). The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature 402: 294–296.
Geerse GJ, van Gurp LC, Wiegant VM, Stam R (2006). Individual reactivity to the open-field predicts the expression of stress-induced behavioural and somatic pain sensitisation. Behav Brain Res 174: 112–118.
Goto Y, Grace AA (2006). Alterations in medial prefrontal cortical activity and plasticity in rats with disruption of cortical development. Biol Psychiatry 60: 1259–1267.
Goto Y, Otani S, Grace AA (2007). The Yin and Yang of dopamine release: a new perspective. Neuropharmacology 53: 583–587.
Grace AA, Bunney BS (1984). The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890.
Gresch PJ, Sved AF, Zigmond MJ, Finlay JM (1994). Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem 63: 575–583.
Hopper JW, Pitman RK, Su Z, Heyman GM, Lasko NB, Macklin ML et al (2008). Probing reward function in posttraumatic stress disorder: expectancy and satisfaction with monetary gains and losses. J Psychiatr Res 42: 802–807.
Houlihan DJ (2011). Psychostimulant treatment of combat-related posttraumatic stress disorder. J Psychopharmacol 25: 1568–1572.
Inoue T, Izumi T, Maki Y, Muraki I, Koyama T (2000). Effect of the dopamine D(1/5) antagonist SCH 23390 on the acquisition of conditioned fear. Pharmacol Biochem Behav 66: 573–578.
Kallai J, Makany T, Csatho A, Karadi K, Horvath D, Kovacs-Labadi B et al (2007). Cognitive and affective aspects of thigmotaxis strategy in humans. Behav Neurosci 121: 21–30.
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 593–602.
Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060.
Kofoed L, Friedman MJ, Peck R (1993). Alcoholism and drug abuse in patients with PTSD. Psychiatr Q 64: 151–171.
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ et al (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131: 391–404.
Langevin JP, De Salles AA, Kosoyan HP, Krahl SE (2010). Deep brain stimulation of the amygdala alleviates post-traumatic stress disorder symptoms in a rat model. J Psychiatr Res 44: 1241–1245.
Liberzon I, Sripada CS (2008). The functional neuroanatomy of PTSD: a critical review. Prog Brain Res 167: 151–169.
Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S et al (1999). Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry 45: 817–826.
Lisman J, Grace AA, Duzel E (2011). A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci 34: 536–547.
Lisman JE, Grace AA (2005). The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46: 703–713.
Louvart H, Maccari S, Ducrocq F, Thomas P, Darnaudery M (2005). Long-term behavioural alterations in female rats after a single intense footshock followed by situational reminders. Psychoneuroendocrinology 30: 316–324.
Mahmoodi M, Shahidi S, Hasanein P (2011). Involvement of the ventral tegmental area in the inhibitory avoidance memory in rats. Physiol Behav 102: 542–547.
Mikics E, Baranyi J, Haller J (2008). Rats exposed to traumatic stress bury unfamiliar objects--a novel measure of hyper-vigilance in PTSD models? Physiol Behav 94: 341–348.
Moaddab M, Haghparast A, Hassanpour-Ezatti M (2009). Effects of reversible inactivation of the ventral tegmental area on the acquisition and expression of morphine-induced conditioned place preference in the rat. Behav Brain Res 198: 466–471.
Moore H, Rose HJ, Grace AA (2001). Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacology 24: 410–419.
Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M et al (2003). Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci 358: 773–786.
Morrow BA, Redmond AJ, Roth RH, Elsworth JD (2000). The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat. Brain Res 864: 146–151.
Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA (2008). Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152: 1024–1031.
Najavits LM, Weiss RD, Shaw SR (1997). The link between substance abuse and posttraumatic stress disorder in women. A research review. Am J Addict 6: 273–283.
Ohl F (2003). Testing for anxiety. Clin Neurosci Res 3: 233–238.
Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L (2007). Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn Mem 14: 861–868.
Payne JD, Nadel L, Britton WB, Jacobs WJ (eds) (2003) The Biopsychology of Trauma and Memory in Humans. Oxford University Press: New York.
Pellow S (1985). Can drug effects on anxiety and convulsions be separated? Neurosci Biobehav Rev 9: 55–73.
Pellow S, Chopin P, File SE, Briley M (1985). Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167.
Pezze MA, Feldon J (2004). Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol 74: 301–320.
Pfeiffer UJ, Fendt M (2006). Prefrontal dopamine D4 receptors are involved in encoding fear extinction. Neuroreport 17: 847–850.
Pissiota A, Frans O, Fernandez M, von Knorring L, Fischer H, Fredrikson M (2002). Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur Arch Psychiatry Clin Neurosci 252: 68–75.
Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I (1996). A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry 39: 129–134.
Rauch SL, Shin LM, Phelps EA (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry 60: 376–382.
Saade NE, Atweh SF, Bahuth NB, Jabbur SJ (1997). Augmentation of nociceptive reflexes and chronic deafferentation pain by chemical lesions of either dopaminergic terminals or midbrain dopaminergic neurons. Brain Res 751: 1–12.
Seip KM, Morrell JI (2009). Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup–but not cocaine-paired contexts. Behav Neurosci 123: 1325–1338.
Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP et al (2004). Hippocampal function in posttraumatic stress disorder. Hippocampus 14: 292–300.
Siegmund A, Wotjak CT (2006). Toward an animal model of posttraumatic stress disorder. Ann N Y Acad Sci 1071: 324–334.
Sigurdsson T, Doyere V, Cain CK, LeDoux JE (2007). Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology 52: 215–227.
Stam R (2007). PTSD and stress sensitisation: a tale of brain and body Part 2: animal models. Neurosci Biobehav Rev 31: 558–584.
Stam R, van Laar TJ, Akkermans LM, Wiegant VM (2002). Variability factors in the expression of stress-induced behavioural sensitisation. Behav Brain Res 132: 69–76.
Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G et al (2012). GABA neurons of the VTA drive conditioned place aversion. Neuron 73: 1173–1183.
Treit D, Fundytus M (1988). Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav 31: 959–962.
Ungless MA, Grace AA (2012) Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons Trends Neurosci.
Valenti O, Lodge DJ, Grace AA (2011). Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci 31: 4280–4289.
Van Dijken HH, Van der Heyden JA, Mos J, Tilders FJ (1992). Inescapable footshocks induce progressive and long-lasting behavioural changes in male rats. Physiol Behav 51: 787–794.
Walf AA, Frye CA (2007). The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2: 322–328.
Yehuda R, Antelman SM (1993). Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry 33: 479–486.
Yehuda R, LeDoux J (2007). Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56: 19–32.
Acknowledgements
We would like to thank Stephen Brookshire, Julia Cremer, and Chu Qin for their technical assistance, Tally Largent-Milnes and Beatriz Fiorivanti for their helpful comments and training, Dr Todd Vanderah for the use of space and technical assistance, and Dr Jose Valdés for all the helpful comments. This work was supported in part by CONACyT (NSCF) and NSF grant CRCNS 1010172 (J-MF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
PowerPoint slides
Rights and permissions
About this article
Cite this article
Corral-Frias, N., Lahood, R., Edelman-Vogelsang, K. et al. Involvement of the Ventral Tegmental Area in a Rodent Model of Post-Traumatic Stress Disorder. Neuropsychopharmacol 38, 350–363 (2013). https://doi.org/10.1038/npp.2012.189
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2012.189
Keywords
This article is cited by
-
Insights into the Involvement and Therapeutic Target Potential of the Dopamine System in the Posttraumatic Stress Disorder
Molecular Neurobiology (2023)
-
Importance of sex and trauma context on circulating cytokines and amygdalar GABAergic signaling in a comorbid model of posttraumatic stress and alcohol use disorders
Molecular Psychiatry (2021)
-
Ventral Tegmental Area Dysfunction and Disruption of Dopaminergic Homeostasis: Implications for Post-traumatic Stress Disorder
Molecular Neurobiology (2021)
-
Rodent models of post-traumatic stress disorder: behavioral assessment
Translational Psychiatry (2020)
-
Diazepam actions in the VTA enhance social dominance and mitochondrial function in the nucleus accumbens by activation of dopamine D1 receptors
Molecular Psychiatry (2018)