Abstract
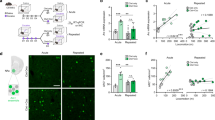
The reconsolidation of cocaine memories following retrieval is necessary for the sustained ability of a cocaine-paired environmental context to elicit cocaine seeking. Extracellular signal-regulated kinase (ERK) is an intracellular signaling molecule involved in nucleus accumbens core (NACc)-mediated reconsolidation of Pavlovian cocaine memories. Here, we used a rodent model of drug context-elicited relapse to test the hypothesis that ERK would be similarly required for the reconsolidation of context-response-cocaine memories that underlie drug context-induced reinstatement of instrumental cocaine-seeking behavior, with a focus on the NACc and on the basolateral amygdala (BLA), another important locus for the reconsolidation of cocaine memories. We show that the mitogen-activated protein kinase (MEK)/ERK1/2 inhibitor, U0126 (1.0 μg/0.5 μl/hemisphere), microinfused bilaterally into the BLA—but not the NACc—immediately after brief re-exposure to a previously cocaine-paired context (that is, cocaine-memory reactivation), significantly attenuated subsequent drug context-induced cocaine seeking relative to vehicle (VEH). This effect in the BLA was associated with a transient inhibition of ERK1/2 phosphorylation, and it depended on memory reactivation given that U0126 administered following exposure to a novel context did not alter subsequent cocaine seeking. Furthermore, similar to U0126, baclofen+muscimol-induced (B+M; 106.8/5.7 ng/0.5 μl/hemisphere) neural inactivation of the NACc, following cocaine-memory reactivation, failed to alter subsequent cocaine seeking. These findings demonstrate that ERK activation in the BLA, but not the NACc, is required for the reconsolidation of context-response-cocaine associative memories. Together with prior research, these results suggest that contextual drug-memory reconsolidation in Pavlovian and instrumental settings involves distinct neuroanatomical mechanisms.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adams JP, Sweatt JD (2002). Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol 42: 135–163.
Berhow MT, Hiroi N, Nestler EJ (1996). Regulation of the ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci 16: 4707–4715.
Brown TE, Lee BR, Sorg BA (2008). The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem 15: 857–865.
Cammarota M, Bevilaqua LRM, Medina JH, Izquierdo I (2004). Retrieval does not induce reconsolidation of inhibitory avoidance memory. Learn Mem 11: 572–578.
Childress A, Ehrman R, McLellan AT, O’Brien C (1988). Conditioned craving and arousal in cocaine addiction: A preliminary report. NIDA Res Monogr 81: 74–80.
Choe ES, McGinty JF (2001). Cyclic AMP and mitogen-activated protein kinases are required for glutamate-dependent cyclic AMP response element binding protein and Elk-1 phosphorylation in the dorsal striatum in vivo. J Neurochem 75: 401–412.
Crespo JA, Stöckl P, Ueberall F, Jenny M, Saria A, Zernig G (2012). Activation of PCKzeta and PKMzeta in the nucleus accumbens core is necessary for the retrieval, consolidation, and reconsolidation of a drug memory. PLoS One 7: 1–9.
Crombag HS, Bossert JM, Koya F, Shaham Y (2008). Context-induced relapse to drug seeking: A review. Philos Trans R Soc Lond B Biol Sci 363: 3233–3243.
Derkinderen P, Enslen H, Girault JA (1999). The ERK/MAP-kinase cascade in the nervous system. NeuroReport 10: R24–R34.
Duvarci S, Nader K, LeDoux JE (2005). Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci 21: 283–289.
Finnie PS, Nader K (2012). The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neurosci Biobehav Rev 36: 1667–1707.
Frankland PW, Bontempi B (2005). The organization of recent and remote memories. Nat Rev Neurosci 6: 119–130.
Fricks-Gleason AN, Marshall JF (2008). Post-retrieval beta-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem 15: 643–648.
Fuchs RA, Bell GH, Ramirez DR, Eaddy JL, Su Z-I (2009). Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci 30: 889–900.
Fuchs RA, Lasseter HC, Ramirez DR, Xie X (2008b). Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov Today Dis Models 5: 251–258.
Fuchs RA, Ramirez DR, Bell GH (2008a). Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 200: 545–556.
Girault JA, Valjent E, Caboche J, Hervé D (2007). ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol 7: 77–85.
Goodman RH (1990). Regulation of neuropeptide gene expression. Annu Rev Neurosci 13: 111–127.
Impey S, Obrietan K, Storm DR (1999). Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron 23: 11–14.
Izquierdo LA, Viola H, Barros DM, Alonso M, Vianna MR, Furman M et al (2001). Novelty enhances retrieval: molecular mechanisms involved in rat hippocampus. Eur J Neurosci 13: 1464–1467.
Kalivas PW, Obrien C (2008). Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology (Berl) 33: 166–180.
Kelly A, Laroche S, Davis S (2003). Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci 23: 5354–5360.
Li F, Xue Y, Wang J, Fang Q, Li Y, Zhu W et al (2010). Basolateral amygdala cdk5 activity mediates consolidation and reconsolidation of memories for cocaine cues. J Neurosci 30: 10351–10359.
Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM et al (2008). Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci 28: 13248–13257.
London SE, Clayton DF (2008). Functional identification of a sensory mechanism required for developmental song learning. Nat Neurosci 11: 579–586.
Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y (2005). Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8: 212–219.
Lu L, Koya E, Zhai H, Hope BT, Shaham Y (2006). Role of ERK in cocaine addiction. Trends Neurosci 29: 696–703.
Martin JH, Ghez C (1999). Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods 86: 145–159.
Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W et al (2002). Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron 34: 807–820.
McClelland JL, McNaughton BL, O’Reilly RC (1995). Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102: 419–457.
Milekic MH, Alberini CM (2002). Temporally graded requirement for protein synthesis following memory reactivation. Neuron 36: 521–525.
Miller CA, Marshall JF (2005). Molecular substrates for retrieval and reconsolidation of a cocaine-associated contextual memory. Neuron 47: 873–884.
Milton AL, Everitt BJ (2010). The psychological and neurochemical mechanisms of drug memory reconsolidation: Implications for the treatment of addiction. Eur J Neurosci 31: 2308–2319.
Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ (2008a). Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci 28: 8230–8237.
Milton AL, Lee JL, Everitt BJ (2008b). Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on beta-adrenergic receptors. Learn Mem 15: 88–92.
Nader K, Schafe GE, LeDoux JE (2000b). The labile nature of the consolidation theory. Nat Rev Neurosci 1: 216–219.
Nolen-Hoeksema S, Stice E, Wade E, Bohon C (2007). Reciprocal relations between rumination and bulimic, substance abuse, and depressive symptoms in female adolescents. J Abnormal Psych 116: 198–207.
Paxinos G, Watson C (1997) The Rat Brain in Stereotaxic Coordinates, Compact 3rd edn. Academic Press: San Diego, CA.
Pedreira ME, Maldonado H (2003). Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38: 863–869.
Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR (2010). Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci 30: 4401–4407.
Sgambato V, Pagès C, Rogard M, Besson MJ, Caboche J (1998). Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci 18: 8814–8825.
Shiflett MW, Martini RP, Mauna JC, Foster RL, Peet E, Thiels E (2008). Cue-elicited reward-seeking requires extracellular signal-regulated kinase activation in the nucleus accumbens. J Neurosci 28: 1434–1443.
Silingardi D, Angelucci A, De Pasquale R, Borsotti M, Squitieri G, Brambilla R et al (2011). ERK pathway activation bidirectionally affects visual recognition memory and synaptic plasticity in the perirhinal cortex. Front Behav Neurosci 5: 84.
Sorg BA (2012). Reconsolidation of drug memories. Neurosci Biobehav Rev 36: 1400–1417.
Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S (2004). Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24: 4787–4795.
Sweatt JD (2001). The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem 76: 1–10.
Théberge FR, Milton AL, Belin D, Lee JL, Everitt BJ (2010). The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem 17: 444–453.
Tronson NC, Taylor JR (2007). Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8: 262–275.
Valjent E, Corbillé AG, Bertran-Gonzalez J, Hervé D, Girault JA (2006). Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci USA 21: 2932–2937.
Valjent E, Corvol J-C, Pagès C, Besson M-J, Maldonado R, Caboche J (2000). Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci 20: 8701–8709.
Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L (2008). Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci 28: 5602–5610.
Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA (2011). Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent durg context-induced cocaine-seeking behavior in rats. Learn Mem 18: 693–702.
Wu P, Xue YX, Ding ZB, Xue LF, Xu CM, Lu L (2011). Glycogen synthase kinase 3β in the basolateral amygdala is critical for the reconsolidation of cocaine reward memory. J Neurochem 118: 113–125.
Zhai H, Li Y, Wang X, Lu L (2008). Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signaling pathway: implications for reinforcement and reinstatement. Cell Mol Neurobiol 28: 157–172.
Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y et al (2004). Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci 24: 3344–3354.
Acknowledgements
We thank Trey Newsome and Christopher Hanlin for outstanding technical assistance and Dr Courtney Miller for providing important protocols. This work was supported by NIDA R01 DA017673, DA025646, T32 DA007244, and F31 DA028051.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Wells, A., Arguello, A., Xie, X. et al. Extracellular Signal-Regulated Kinase in the Basolateral Amygdala, but not the Nucleus Accumbens Core, is Critical for Context-Response-Cocaine Memory Reconsolidation in Rats. Neuropsychopharmacol 38, 753–762 (2013). https://doi.org/10.1038/npp.2012.238
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2012.238
Keywords
This article is cited by
-
Drug memory reconsolidation: from molecular mechanisms to the clinical context
Translational Psychiatry (2023)
-
Modulation of methamphetamine memory reconsolidation by neural projection from basolateral amygdala to nucleus accumbens
Neuropsychopharmacology (2023)
-
Neuroplastic Changes in the Superior Colliculus and Hippocampus in Self-rewarding Paradigm: Importance of Visual Cues
Molecular Neurobiology (2022)
-
Silent synapses dictate cocaine memory destabilization and reconsolidation
Nature Neuroscience (2020)
-
Molecular and circuit mechanisms regulating cocaine memory
Cellular and Molecular Life Sciences (2020)